One-Step HCV RNA Tests Help Encourage Hepatitis C Screening
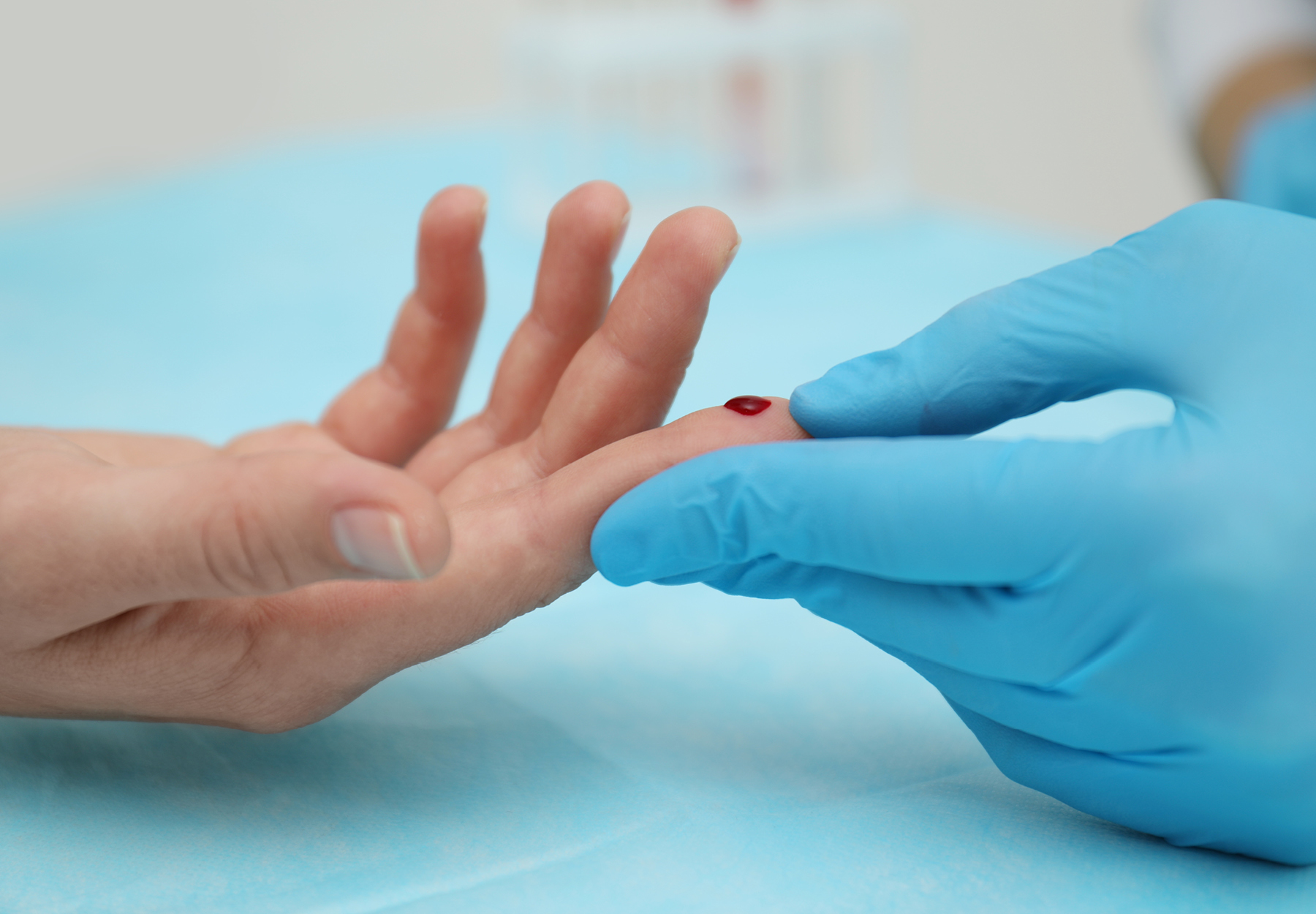
Viral hepatitis is responsible for 1.34 million worldwide deaths each year, as many as HIV/AIDS, tuberculosis, or malaria. The World Health Organization (WHO) has recognized the critical role played by laboratory diagnostics in eliminating the hepatitis C virus (HCV) by 2030. Onsite HCV screening using fingerstick technology offers the promise of rapid, cost-effective, and accurate diagnosis at the point of care. The Diagnostic Challenge Conventional HCV testing requires patients to make multiple clinical visits. During the first visit, clinicians perform a test for HCV antibodies. A positive test result means only that the patient has been exposed to the virus. The patient must then schedule another visit to be tested for HCV RNA to determine the presence of active infection. Sometimes subsequent visits are necessary. All of this requires laboratory analysis, trained phlebotomists, sample preparation, and other test resources. And in many cases, patients who test positive in visit one do not follow up with a second visit. New products from companies like Cepheid, OraSure, and Abbott Laboratories enabling detection of active HCV infection in a single step may be much more effective in engaging patients and convincing them to get screened. There are two basic kinds of HCV tests: […]
Subscribe to Clinical Diagnostics Insider to view
Start a Free Trial for immediate access to this article