Beyond the Pap Smear: New Methods to Improve Cytological Screening
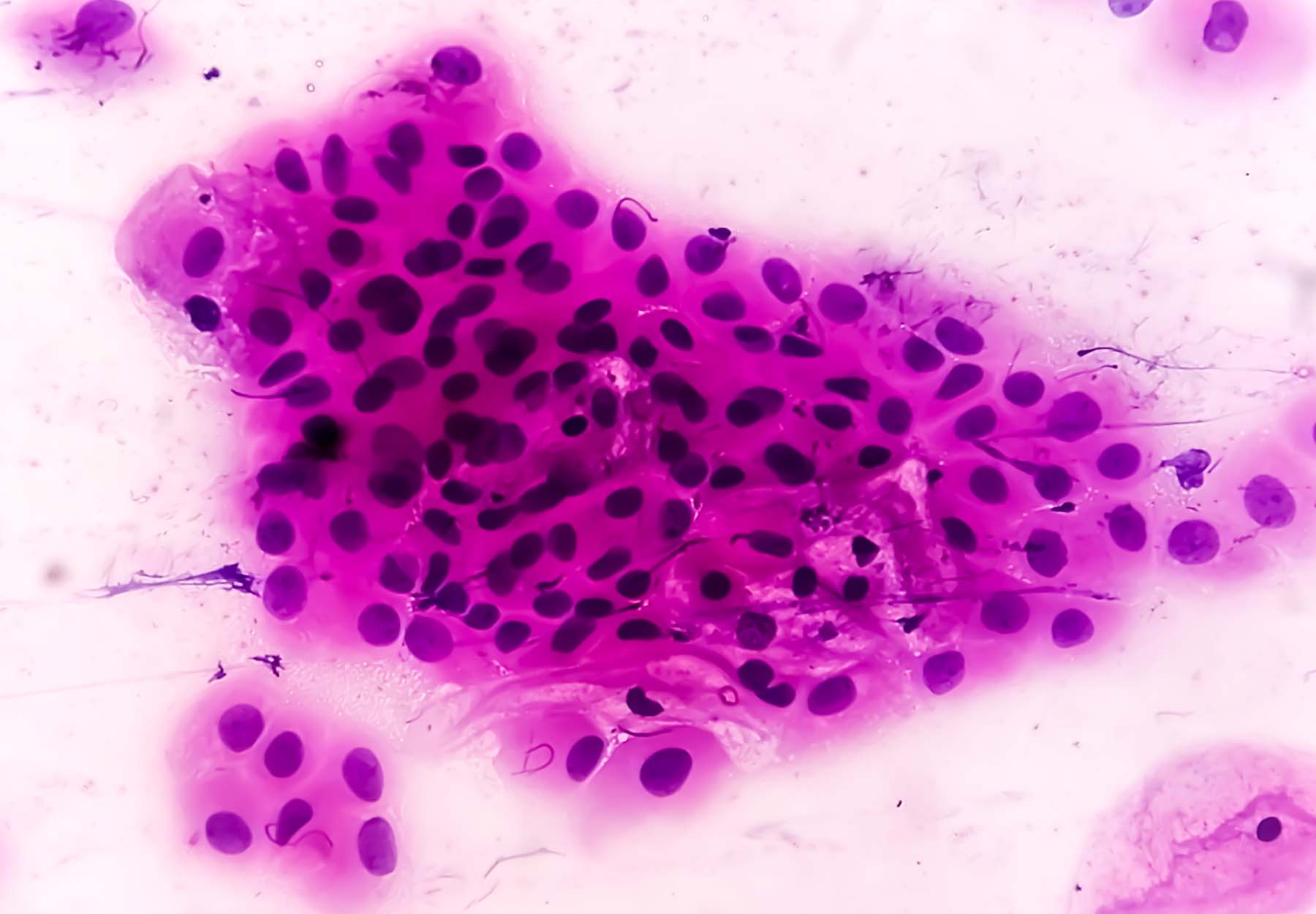
According to American Cancer Society (ACS) estimates, roughly 14,140 new cases of invasive cervical cancer will be diagnosed in the US in 2022; and about 4,280 women will die from cervical cancer. These sobering numbers belie the fact that cervical cancer is highly preventable. Conventional cytology screening methods, i.e., Papanicolaou or “Pap” tests, have proven extremely effective in reducing cervical cancer incidence and mortality. But they also require the use of analytical microscopy methods that are prone to yielding inaccurate results. The good news is that researchers and test makers are making significant progress toward developing new and more cost-effective screening methods that can be deployed at the point of care. Pap Testing Pros & Cons The Pap smear is a non-invasive cytologic screening test performed by scraping cells from the cervix, fixing them onto a glass slide, and examining them under a microscope. Because the Pap test identifies precancerous cells or cellular changes in the cervix before they become invasive tumors, it is valuable for screening and early detection, which can improve case outcomes and save lives. Widespread adoption of Pap tests in clinical practice has reduced cervical cancer death rates by nearly 50 percent in American women. However, […]
Subscribe to Clinical Diagnostics Insider to view
Start a Free Trial for immediate access to this article