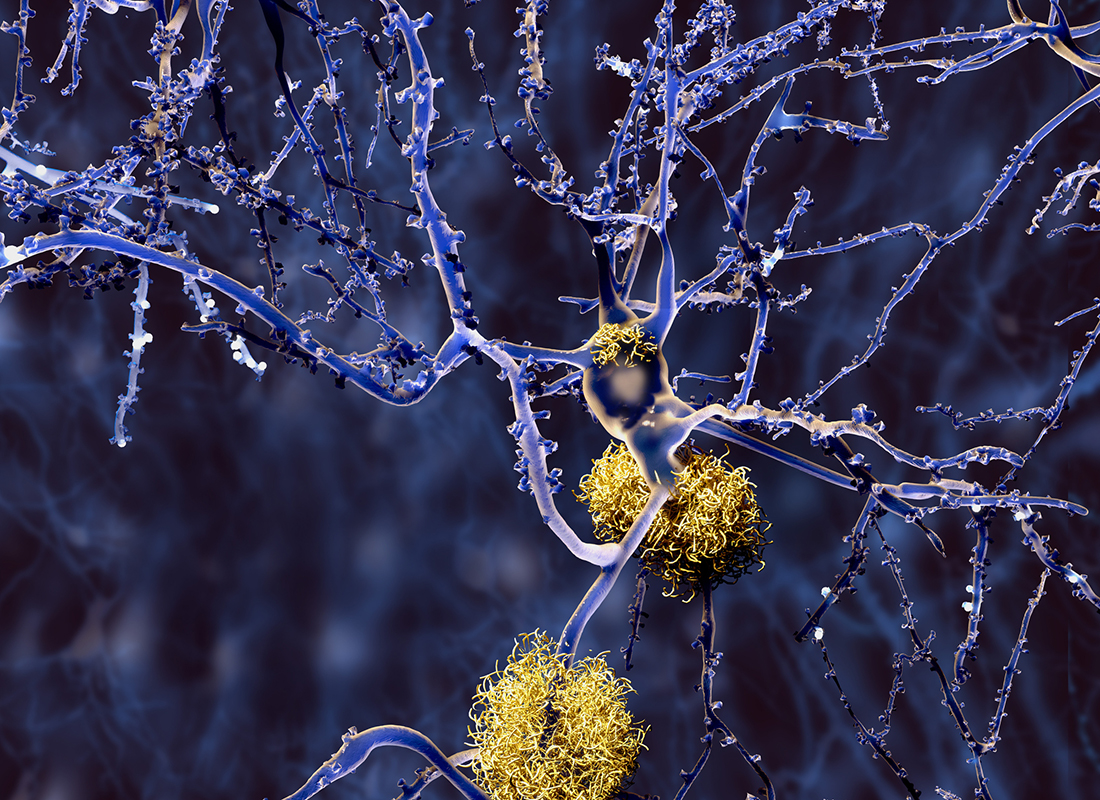
Can Ophthalmic Biomarkers Offer Insights into Brain Pathologies?
Protein levels in the vitreous humor may have the potential to support the diagnosis and staging of neurodegenerative diseases.
Subscribe to Clinical Diagnostics Insider to view
Start a Free Trial for immediate access to this article