December 2022 FDA Watch: COPD, COVID-19, and MDD Tests Gain Clearance
A roundup of the significant new diagnostic tests and products that received regulatory approval in the US and around the world.
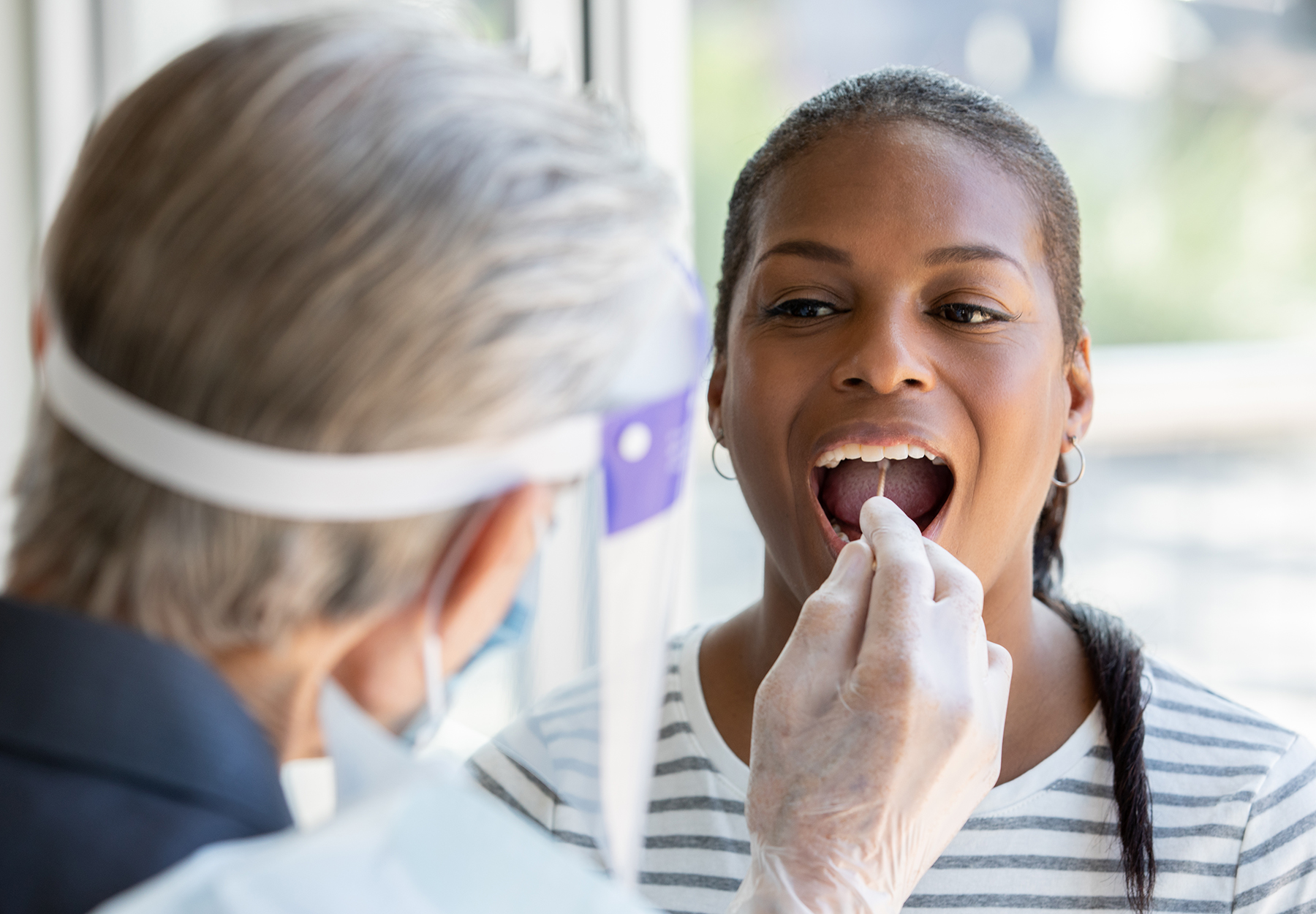
Here are three of the most significant new diagnostics tests and products that gained regulatory clearance from the FDA and the European Union (EU) in the past month.
1. Grifols Gets FDA Clearance for DTC Use of COPD-Related Assay
The FDA cleared Grifols’ AlphaID cheek swab test for alpha1-antitrypsin (AAT) deficiency for direct-to-consumer (DTC) use.1 AAT deficiency is an inherited disorder and common risk factor for chronic obstructive pulmonary disorder (COPD). In 2019, the agency gave the green light to market the test for use with a prescription. The newly expanded clearance allows for use of the test with saliva samples collected at home with OraSure’s ORAcollect Dx collection device, which had previously been available via prescription only.2
2. FDA Clears First OTC At-Home Saliva Test for COVID-19
On Oct. 18, the FDA broke new ground by issuing the first Emergency Use Authorization (EUA) for an over-the-counter (OTC) saliva-based COVID molecular test to Aptitude Medical Systems for the Metrix COVID-19 Test, a single-use test that uses real-time loop-mediated isothermal amplification (RT-LAMP) with a fluorescence reader to detect the SARS-CoV-2 virus.3
Context: The standard method of detecting upper respiratory viruses like SARS-CoV-2 is to test tissue samples from the back of the sinus cavity collected by inserting a long nasopharyngeal swab deep into the nostril. Testing on saliva simples is easier, faster, and more comfortable. However, saliva-based tests for COVID-19 have been largely a sideshow. Early in the public health emergency (PHE), Rutgers University scored the first EUA for a saliva COVID-19 test, the Rutgers Clinical Genomics Laboratory TaqPath SARS-CoV-2 Assay test which runs on the Thermo Fisher Applied Biosystems QuantStudio 5 Real-Time PCR System. More than 30 saliva-based kits have received EUA during the PHE, many for use at the point of care.
3. Genetika+ Gets European Clearance for MDD Personalized Treatment Test
Israeli startup Genetika+ made big news in Europe by securing CE-IVD marking for NeuroKaire, a blood-based, artificial intelligence-driven test for predicting a major depressive disorder (MDD) patient’s response to different antidepressant treatments. The combination of patient-reported symptoms, pharmacogenetics testing, and functional testing of the blood sample enables physicians to personalize and design optimal treatment for individuals suffering from MDD. The results of the validation study “confirm the utility of our brain-in-a-dish technology, reflecting the individual patient’s response to the drug in the target organ,” noted Genetika+ CEO and co-founder Dr. Talia Cohen Solal in the press release announcing the approval.4
References:
- https://www.prnewswire.com/news-releases/grifols-receives-fda-clearance-for-alphaid-at-home-the-first-free-service-for-us-consumers-to-determine-their-risk-for-alpha-1-301667630.html
- https://orasure.gcs-web.com/news-releases/news-release-details/orasure-technologies-receives-general-clearance-its
- https://www.fda.gov/media/162401/download
- https://www.prnewswire.com/il/news-releases/genetika-announces-ce-mark-for-neurokaire-its-ai-powered-tool-to-optimize-depression-treatment-301653411.html
****
Here are some of the other key new FDA EUAs and clearances that were announced from late October through mid-November 2022:
New FDA Approvals & Emergency Use Authorizations (EUAs)
| Manufacturer(s) | Product |
|---|---|
| Roche | Premarket approval for Cobas 5800 compact PCR molecular diagnostics system and assay to detect HIV-1 |
| Roche | 510(k) clearance for its Cobas SARS-CoV-2 Qualitative PCR test which had previously received only EUA clearance |
| Grifols | Clearance for direct-to-consumer use of AlphaID, cheek swab-based test for alpha1-antitrypsin (AAT) deficiency |
| OraSure Technologies | Clearance for over-the-counter use of ORAcollect Dx collection device to support screening using Grifols' AlphaID test for AAT deficiency |
| 23andMe | 510(k) clearance for pharmacogenetics report for SLCO1B1 that includes interpretive drug information for simvastatin |
| BioMérieux | 510(k) clearance for Vitek 2 AST-Gram Positive Cefoxitin Screen, fully automated short-term incubation cycle antimicrobial susceptibility test for use on firm's Vitek 2 and Vitek 2 Compact systems |
| BioFire Diagnostics (BioMérieux subsidiary) | Clearance for BioFire Global Fever Panel, multiplex assay designed for use with BioFire FilmArray 2.0 and FilmArray Torch PCR system |
| Abbott | Clearance for Architect CMV IgG test, a chemiluminescent microparticle immunoassay to measure IgG antibodies |
| Helena Laboratories | Clearance for Spife A1AT Kit, an alpha-1 antitrypsin immunological test |
| Instrumentation Laboratory (Werfen subsidiary) | Clearance for HemosIL Liquid Anti-Xa test, automated chromogenic heparin assay with synthetic chromogenic substrate and Factor Xa inactivation |
| CoaguSense | Clearance for Coag-Sense Prothrombin Time (PT)/INR Monitoring System for Patient Self Testing |
| Nova Biomedical | 510(k) clearance for Nova Primary Glucose Analyzer System, enzymatic blood-glucose concentration test system |
| OK BioTech | 510(k) clearance for SuperCheck Pro Blood Glucose Monitoring System |
| Sol Millennium | 510(k) clearance for Sol-Guard Safety Pull Button Blood Collection Set spring-activated needle retraction device |
| Aptitude Medical | EUA for Metrix COVID-19 Test, first COVID-19 single-use saliva test to receive approval for over-the-counter home use |
New CE Marks & Global Certifications
New CE Markings in Europe
| Manufacturer(s) | Product(s) |
|---|---|
| Cepheid | Xpert Xpress GBS group B Streptococcus assay |
| FireGene | Monkeypox virus nucleic acid test kit |
| Genetika+ | NeuroKaire AI-powered blood test enabling physicians to personalize treatment for major depressive disorder (MDD) |
| MedMira | Vyra COVID-19 Antigen Test |
Other international clearances announced during the period:
| Manufacturer(s) | Country(ies) | Product(s) |
|---|---|---|
| Novacyt | UK | Winterplex 3G real-time PCR assay for SARS-CoV-2, influenzas A and B, and respiratory syncytial virus |
Subscribe to Clinical Diagnostics Insider to view
Start a Free Trial for immediate access to this article