The FDA continues to sound the warning on unauthorized SARS-CoV-2 rapid antigen tests. On March 1, the agency issued a trio of Safety Communications warning providers and consumers not to use:
- The Celltrion DiaTrust COVID-19 Ag Rapid Test—the one that comes in a green and white box and has the same name as another test in different packaging that has received authorization;

The Celltrion DiaTrust COVID-19 Ag Rapid Test that comes in a green and white box. Image provided by the FDA.
- The SD Biosensor Inc. STANDARD Q COVID-19 Ag Home Test; and
- The ACON Laboratories Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing), which comes in a dark blue box, as opposed to the differently packaged ACON Laboratories Flowflex COVID-19 Antigen Home Test, which has received emergency use authorization and isn’t the subject of the FDA warning.
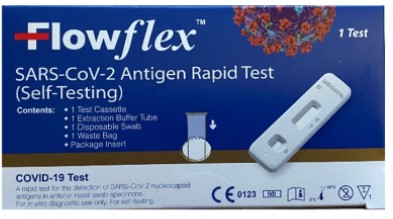
The ACON Laboratories Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing), which comes in a dark blue box. Image provided by the FDA.
Not even three months into the year, the FDA has issued six do-not-use warnings for eight different unauthorized SARS-CoV-2 rapid tests, including assays from LuSys Laboratories, Empowered Diagnostics, and E25Bio.
See more on recent recalls in the March 2022 issue of Diagnostic Testing & Emerging Technologies.
