Using a printer to create tissues sounds like like science-fiction, but researchers are making rapid progress in the field of bioprinting. While printing off replacement organs for human transplantation may be years off, early applications of bioprinted tissues are currently being used in pharmaceutical development and academic research laboratories for biomarker discovery, drug screening, and toxicology testing. Bioprinted tissues may also reach clinical laboratories in the not-so-distant future.“The concept of bioprinting, which is essentially an extension of the philosophy that uses additive manufacturing methods to build complex scaffold structures, can be thought of as a combination of (i) different types of cells in defined locations, (ii) supporting matrix or scaffolds (if required), and (iii) biochemical cues to control behavior,” writes Brian Derby, Ph.D., from the University of Manchester in a review piece published in the Nov. 16 issue of Science. “Although bioprinting has its origins in the area of tissue engineering and is sometimes described as organ printing, there are other application areas where a printed or artificially fabricated tissue analog structure is useful, including cell-based sensors, drug/toxicity screening.”
How It Works
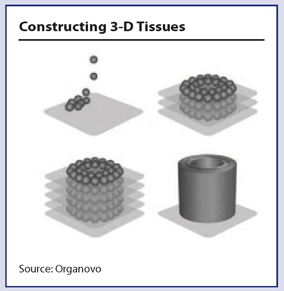
Progress in bioprinting is happening at the intersection of biotechnology and manufacturing. It is based on the computer-controlled delivery of cells into three-dimensional constructs.
Progress in the field has been rapid since Gabor Forgacs, Ph.D., at the University of Missouri, Columbia, made the seminal discovery in 1996 that during embryonic development, cells clump together with liquidlike properties. In 2003 Thomas Boland, Ph.D., then at Clemson University in South Carolina, modified an inkjet printer to dispense cells into scaffolds. Since then researchers have developed technologies allowing 3-D tissues to be engineered without scaffolding. The first commercial 3-D bioprinter was developed in 2009 by a bioprinting company called Organovo (San Diego). Forgacs’s discoveries are the foundation for Organovo’s technology.
Cells, collected from the patient or stem cells, are cultured and then used to create a bio-ink, which is loaded into a cartridge. The printing process uses layers of an inert hydrogel support matrix and the bio-ink, which allows for control of cell distribution. Droplets of bio-ink measure 100 microns to 500 microns in diameter and contain more than 10,000 cells each. The computer-controlled printer heads are programmed and layering is repeated in a specified geometry and the cells naturally fuse together in a biocompatible form. Current research efforts are aimed at increasing the diversity of cell lines used in bio-ink and improving the cell viability during the printing process.
The resulting tissue has tremendous biological potential and is superior to animal models, researchers say, because it is made out of human cells. These tissue models have the potential to replace two-dimensional cell-based arrays for studying absorption/distribution/metabolism/excretion (ADME) models, toxicology, and drug metabolism and pharmacokinetics.
Current Applications
Organovo is using the 3-D human tissue printing technology to create tissue on demand. The company’s tissues are being used to recapitulate in vivo biology for human disease research, drug discovery and development, toxicology testing, and, eventually, as therapeutics themselves.
“The big challenge in the pharmaceutical industry and academic labs is the lack of a robust preclinical model,” says Eric David, M.D., chief strategy officer at Organovo. “They are surprised too often with a failure in the late stages—in late 2 or stage 3 trials after they have spent $600 million to $1 billion. You can’t have failure at that stage for efficacy or safety.”
“Bioprinting is so valuable because it is an architecturally correct, fully human model.” —Eric David, M.D.
David tells
DTTR that bioprinting can play an important role in improving the predictiveness of preclinical models, even better than animal models.
“Animal models are a whole organism, but they are not human. Cells in petri dishes are two-dimensional and don’t behave like normal human cells,” explains David. “Bioprinting is so valuable because it is an architecturally correct, fully human model.”
The company’s tissues are currently being used in drug discovery research under collaborative research agreements with Pfizer and United Therapeutics, as well as in academic research at Harvard Medical School and the Sanford Consortium for Regenerative Medicine. David says the technology is not used for super high-throughput applications like screening 10,000 compounds, but once the list is whittled down to a dozen or so targets, the model works.
Over the summer the company received its first company patent for multilayered vascular tubes as well as a key founder patent for core bioprinting technologies assigned to the University of Missouri and exclusively licensed to Organovo. The company also moved into larger facilities with three times the laboratory space and approximately four times the cleanroom space of our prior facility, which will provide capacity for near-term product manufacturing needs.
Organovo, David says, is not looking to be in the business of selling the instruments and the consumables, but rather to sell the tissues on 96-well plates or in bigger tissues constructs on 24-well plates. Within a year he sees the company as having “off-the-shelf” tissue options including for researchers testing liver toxicity to research in vivo drug interactions. The earliest successes for the bioprinted tissues will likely be in fibrotic diseases and in liver and kidney disease, which currently have poor disease models. David says he could see uses for bioprinted tissues in clinical laboratories as well in the future—better enabling comparative histological examinations. “Biobanking only goes so far,” he says.
Organovo’s bioprinting technology is also being used to develop tissues for direct therapy, such as cardiac muscle patches, although the company is not yet actively in clinical trials.
“We are still a long way from organ printing. Although current deposition and fabrication technologies allow us to build structures that are analogous to tissue in their composition, the development of fully functioning tissue is a much greater step,” writes Derby in the review. “There is also still considerable uncertainty concerning the level of cell damage that occurs during cell deposition by all bioprinting methods. It is clear that much further work will be needed in this area before regulatory approval can be obtained for translational studies.”
Regulatory challenges seem to pose significant concern for researchers involved in biomaterials research. In a special issue focused on regenerative biotissue, experts commented on some of the challenges associated with translating this emerging research into clinical applications.
Bioresponsive materials that boost the maturation and differentiation of therapeutic cells may be treated as combination products requiring regulatory approval of the material or device as well as the cellular components, says Alan Tounson, Ph.D., in comments in the Nov. 14 special issue of
Science Translational Medicine.
“Drugs and medical devices follow a refined preclinical testing framework: animal models, study designs, statistical plans, and diagnostics practically comprise a ‘cookbook,’ which facilitates the risk-benefit analysis process for all reviewers,” says Tounson, president of the California Institute for Regenerative Medicine. “There is no such cookbook for biomaterials with biologic components.”
“Although current deposition and fabrication technologies allow us to build structures that are analogous to tissue in their composition, the development of fully functioning tissue is a much greater step.” —Brian Derby, Ph.D.
“Long development timelines, funding shortages, and regulatory uncertainty hinder the clinical translation and commercialization process for combination biomaterials,” adds Chris Mason, M.D., Ph.D., from the Advanced Centre for Biochemical Engineering at the University College London, in the same piece. “Investors and investigators alike focus on removing complexity, by going with either cells or the biomaterial alone, to ease the regulatory burden and reduce uncertainty. Unfortunately, this strategy is in opposition with the diverse range of unmet clinical needs (many of which will not be solved by one technology alone) and the ability of the field to achieve its full potential through the development of multifunctional combination materials.”
For the near-term Derby says that it is likely these tissue analog structures will be limited to applications such as toxicity screening and drug testing, and to construct tumor models, allowing variation in physiological conditions in vitro.