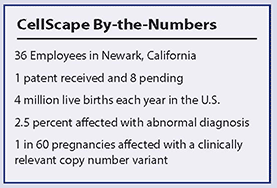
CellScape (Newark, Calif.) is poised to enter the rapidly growing noninvasive prenatal diagnostics market in the second half of 2014 with its Clarity Prenatal Test. Unlike other currently marketed tests, Clarity relies on whole fetal cells and targeted microarray technology to detect roughly 30 chromosomal abnormalities (microdeletion/insertion syndromes) beyond common aneuploidies (Trisomies 21, 18, and 13), making the tests’ results capabilities the closest to invasive testing like amniocentesis, the gold standard, in terms of genetic coverage.
Acknowledging a competitive market space and reimbursement challenges, CellScape has strategically positioned itself with its patented fetal cell separation methodology, enabling the company to initially enter the market by partnering with existing reference and academic labs.
DTET recently spoke with CellScape’s CEO Ted Snelgrove, who joined the company in July, and co-founder and executive chair Karen Drexler regarding the development and launch of Clarity as well as the future of noninvasive prenatal testing.
What differentiates CellScape’s approach from other companies’ in the noninvasive testing space?
Drexler: We had looked at the other approaches and what everybody else had attempted to do in this field. Because our team is an engineering team, rather than out of traditional biology, we took a very different approach. The standard techniques available in labs—various molecular enrichment techniques, magnetic cell sorting, and fluorescent cell sorting—have the goal to reduce down the population of cells from 100 billion cells, which you have in two tubes of blood. Everybody else tried to get down to 1 million to 3 million cells, which is about what you can view on one or two or three microscope slides. We, however, decided we wanted to maximize retention of nucleated cells, which have DNA.
In our first enrichment step, we only eliminate non-nucleated red blood cells. At the end of our enrichment process, which is a proprietary process, we are left with about a billion cells. Using advances in hardware and software, tools that are more often used for defect recognition, like on silicon wafers, we are able to isolate cells of interest through these optical techniques versus using physical cell separation. It has really made a difference.
Why did CellScape choose to use microarray technology rather than next-generation sequencing?
Drexler: We are a sample prep company primarily. Our goal is to retain high-quality fetal cells that can be applied to whatever kind of genetic analysis is most appropriate. That is what we do uniquely. We initially thought we would use fluorescence in situ hybridization (FISH) because the market was addressing whole-chromosome abnormalities and we could visualize and count whole chromosomes beautifully with FISH. The key driver of our decision to move to microarray was Ronald Wapner’s NIH [National Institutes of Health]-funded study. That study proved that you could identify a large number of additional pathogenic, important disorders in utero by using chromosomal microarrays versus karyotype. Dr. Wapner presented his findings in February 2012 and culminated in a series of articles published in the
New England Journal of Medicine in December 2012. Once the data was published, it was evident that microarrays would become the gold standard in the marketplace.
We decided to go to the market with a genetic analysis method that was proven, that was understood by physicians and genetic counselors, and that would be used as the standard for detecting a broad range of genetic abnormalities. We will enter the market with a change in sample—using a needle in the mother’s arm rather than a needle in her belly to draw fluid containing fetal cells. At leading labs around the country, chromosomal microarrays either are or are becoming the standard of care. We want to leverage that movement in the market and launch with a novel approach to the sample, but not with changes in the method with respect to the analytical testing.
We also studied sequencing and we do not believe sequencing is the best method today for looking at a large number of copy number variants. It has been done in academic settings, but it costs too much and takes too long to be practical for commercial testing at this time. And the market is not familiar with sequending for prenatal genetic syndromes. Our work in terms of test validation is a lot easier with microarrays and the market understands the use of chromosomal microarrays to assess copy number variants.
What is the process by which CellScape will decide to add a variant to Clarity?
Drexler: This is an area where we want to follow what the market had decided, as opposed to leading the market. We want to incorporate elements into our test that the industry agrees should be tested for prenatally. We are looking to outside experts to help us make those decisions. We have not finalized design of our commercial array yet, but the process we have used is very, very thorough. We started out with all of the data from the Wapner study—all of the chromosomal microarray findings—and looked at those things with pathogenic connections. We are using a targeted microarray that focuses for development purposes on the 30 or so most prevalent defects that are highly penetrant and are very serious affecting the quality of life in the first couple years of life. We are not looking at genetic findings that are associated with disease later in life. We are not looking at traits that are not associated with poor health outcomes. We are only looking at genetic syndromes in which there is a significant impact early on.
We started with the Wapner study data. A geneticist on our team compiled a detailed summary syndrome by syndrome to get the best understanding of the impact of each condition and help us rank order the most serious ones for us to prioritize. We focused on those well-characterized genetic conditions where there is consensus among industry leadership about testing prenatally.
We expect that relevant scientific societies, like ACMG (American College of Medical Genetics), will come out with recommendations on these issues. We are trying to be conservative in this regard. In addition, we just kicked off quantitative market research. We are looking to understand which genetic findings physicians are comfortable counseling patients on. We are working to combine published information and what industry associations designate with clinician input.
On the business side, noninvasive prenatal testing is a competitive marketplace. How is the company positioning itself strategically ahead of launch?
Snelgrove: Since 2001 I’ve been in the broader field of advanced molecular diagnostics. My emphasis has been building strong products with great brand equity that have become reference points in their medical specialties where they were developed—in oncology with Oncotype DX (Genomic Health) and in rheumatology with Vectra DA (Crescendo Bioscience). There is a great opportunity with the Clarity system to build a new brand and to do so at the high end of the market in terms of value proposition. I think there is an opportunity to come in and help define a market like this as noninvasive prenatal genetic testing is still in an early stage. CellScape will enter the market as a follower in the sense that there are marketed services already in this market. However, they all rely on cell-free DNA. From another perspective we are also a first mover because we will introduce noninvasive testing with a whole-cell approach. We are a hybrid in that regard.
As this market matures, it has the potential to grow to 4 million tests per year in the United States alone, and it is potentially much larger than that if you look at the rest of the developed world. Segments may emerge over time. There will be an opportunity for Clairty to become the preferred choice for those who want to get as close to amniocentesis as possible, without having to undergo an invasive procedure. The goal will be to replicate the information from an amnio as much as possible without the risk. That’s a somewhat different value proposition than other companies have and it will differentiate Clarity.
Reimbursement remains an issue throughout the noninvasive prenatal testing market. How do you see reimbursement impacting further adoption?
Snelgrove: We are sorting out the degree to which the advent of the new exchanges will change the market. Medicaid currently pays for a large share—nearly half—of all maternity care in the United States. As the Affordable Care Act is rolled out and the market reaches a new equilibrium, with some groups shifting to expanded Medicaid coverage, the reimbursement landscape will change. It is an open question at the moment that hasn’t been resolved, but over time, I believe, any payer, including the governmental bodies with responsibility for these programs, will agree to provide access to genetic testing for all women who are pregnant, not just those of advanced maternal age, given the real risks for genetic abnormalities across all pregnancies.
The reason adoption was so fast for cell-free testing and the reason reimbursement happened so much more quickly than for any other diagnostic market in recent history is because of strong interest among the women themselves. There is more of a willingness to pay at the patient level than in any other market I’ve seen. While I am not expecting this in the long term to be a patient-paid service, it still means a lot that patients believe it should be covered.
Associated Data