Call it cloudy with a chance for shortfalls. That might be the best way to describe final 2013-2014 gap-fill pricing on dozens of molecular tests released by the Centers for Medicare and Medicaid Services (CMS) on Sept. 30.
While the numbers contained some hope for the sector and its efforts to lift some prices, some test codes received no definitive pricing at all. And some key tests appeared to be priced dramatically lower in some regions than in others—the apparent result of clerical errors by two of the agency’s Medicare administrative contractors (MACs) that left industry observers scratching their heads.
A report by Piper Jaffray said pricing errors were included for BRCA testing in the regions overseen by Noridian and Palmetto, dropping them more than 48 percent than the previous pricing. That would have proven disastrous for Myriad Genetics, the Salt Lake City-based lab that currently performs most of the nation’s BRCA testing and relies on the assay for a large part of its revenue.
“Myriad is working with CMS to rectify the erroneous reimbursement information and anticipates corrected rates will be published in the next week or two,” Piper Jaffray said in a report.
A statement issued by the American Clinical Laboratory Association appeared to confirm Piper Jaffray’s concerns. The organization was “still in the process of reviewing the information that was published . . . but has received several credible reports that there may be clerical errors in the published information that could impact the final pricing for these tests.”
Whether CMS has acknowledged or will fix the apparent errors anytime soon remains to be seen. The federal government entered into a massive shutdown on Oct. 1, furloughing thousands of nonessential employees, with many CMS officials among them.
As a result, calls to the CMS press office went into a closed-loop recording that prevented leaving messages. An e-mail to the CMS spokesperson who handles these specific payment issues generated an automated response saying she is on furlough and unable to respond.
65 Codes Priced
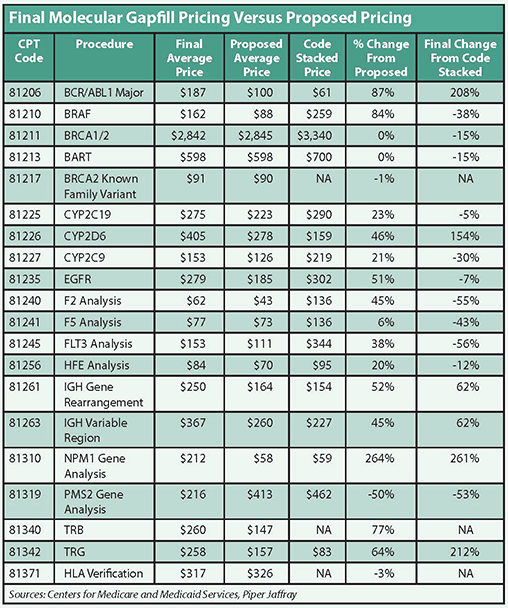
Just hours before the shutdown, the agency issued prices for 65 of the 114 codes that were placed on the Clinical Laboratory Fee Schedule earlier this year, replacing the prior code-stacking methodology. For those codes, prices rose an average of 26 percent and a median of 6 percent compared to the proposed prices that had been issued earlier this year.
Altogether, 39 tests received an average nationwide price boost over their proposed price, while 19 saw cuts and six were unchanged. Thirteen tests saw their final prices increased over what would have been paid using code-stacking methodology, while 24 were cut, most by double-digit percentages.
Pricing released by Palmetto GBA, the MAC with jurisdiction over California and Nevada, led to increases of prices on 43 tests and price reductions on 22 others that ranged from nominal to as high as 81 percent.
Some of the tests received pretty dramatic boosts over the proposed prices that had been released in the spring.
NPM1 gene analysis, CPT code 81310, was priced at a national average of $212, up 264 percent from the average proposed price of $58. It was also up 261 percent from the original code-stacked price of $59. It also fared well in California and Utah, where Palmetto GBA serves as the MAC. It priced the test at $249, up 327 percent from the original proposed price.
Chimerism analysis, CPT code 81268, was priced at an average of $312, up 102 percent from the original proposed price of $150. However, it still fell far short of the original code stacking price of $1,109.
Another test that fared well from its proposed to final average price was MLH1 gene analysis familial variant, CPT code 81293. It originally had a proposed price of $91 but came in at $240, a bump of 165 percent. It also fared well in code stacking, where Medicare originally paid $94, representing a bump of 156 percent.
Although industry experts were still poring over the codes as of earlier this week, there were tacit admissions that they had prevailed on lifting some of the prices.
Some Improvement
“The bottom line is there was some improvement,” said Michael Arnold, president of the California Clinical Laboratory Association, one of the most vocal of the regional lobbying groups on the molecular coding issue. However, Arnold added that he would have liked to see the prices rise even further.
Not every code emerged unscathed. An MSH2 full sequence gene analysis test, CPT code 81295, was priced at $292, a 64 percent reduction from the proposed price of $809 and a 60 percent drop from the code-stacked price of $730. Palmetto cut pricing on that assay 81 percent from its original proposal, down to $153.
Meanwhile, some industry experts have expressed concern about what did not receive specific prices: 51 separate tests. They include ASPA gene analysis, CPT code 81200, which was priced at $213 under code stacking, CFTR gene analyses, CPT codes 81220 to 81224, and long QT gene analysis, CPT codes 81280 to 81282.
“We are extremely concerned about the apparent lack of coverage for many of these tests, despite their current use in patient management, the availability of medical literature supporting those uses, and their inclusion in practice guidelines. In many cases, the results and interpretations provided in the reports of these medically necessary tests are used to determine patient treatment,” said Stephen Black-Schaffer, M.D., a Massachusetts pathologist who is vice chairman of the economic affairs committee of the College of American Pathologists. “CAP is concerned that many proposed contractor coverage decisions would deny beneficiaries’ access to molecular testing that is necessary for their diagnosis and management.” He added that the American Medical Association is reporting denials in Indiana, Texas, Tennessee, Ohio, Kentucky, and Wisconsin.
Arnold also expressed concerns about tests being left off the schedule. “It’s a giant question mark we have,” he said.
Takeaway: The laboratory industry prevailed in some instances to raise prices on dozens of molecular test codes, but errors by the Centers for Medicare and Medicaid Services has clouded just how wide-ranging its successful campaign was.
Associated Data