Hospital-acquired infections (HAIs) are becoming the greatest threat to health care safety in the United States.
According to the Centers for Disease Control and Prevention (CDC), about 2 million incidents of HAIs are reported every year—a number private entities such as the Committee to Reduce Infection Deaths believe is underreported. HAIs also lead to about 100,000 patients dying each year. Each infection costs on average about $20,000 to treat regardless of outcome, and about 70 percent of HAIs are resistant to at least one form of antibiotic.
Moreover, cases of a hospital-acquired intestinal infection known Clostridium difficile, or C. diff., are rapidly growing in the hospital setting. Forms of the bacteria cannot be checked with hand washing and often attack patients after they have been treated with antibiotics for another infection (which reduces the levels of intestinal bacteria that keep C. diff in check). The CDC estimates that as many as 165,000 cases of C. diff. are occuring in hospitals annually, killing 9,000 patients.
With G2 Intelligence’s recent Volume to Value conference in Fort Lauderdale, Fla., exploring ways laboratories can contribute to the productivity of providers, their role in infection control was a natural topic for discussion.
Frederick Kiechle, M.D., medical director of clinical pathology at Pathology Consultants of South Broward in Hollywood, Fla., and Denise L. Uettwiller-Geiger, Ph.D., director of laboratory services at John T. Mather Memorial Hospital in Port Jefferson, N.Y., both spoke of their experiences using laboratory services to battle HAIs.
Molecular Testing for MRSA
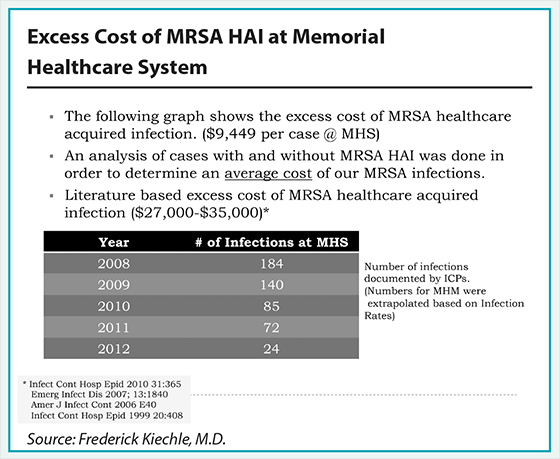
Kiechle works closely with the five-hospital Memorial Healthcare System in the Fort Lauderdale area. In late 2008, Memorial rolled out a system to detect and combat methicillin-resistant Staphylococcus aureus, an HAI better known as MRSA. It represents about 60 percent of all HAIs, and is usually spread via the hands of hospital employees involved in health care delivery. The health system performs testing on high-risk patients—those admitted to or transferred from the intensive care unit, all orthopedic and neurological implant cases, all patients undergoing open heart surgery, and all patients with a prior history of having a MRSA infection.
“It’s not everybody,” Kiechle said, noting that not every patient needs to be tested for the infection.
Memorial uses molecular testing from swabs in a patient’s nasal cavity to detect the presence of MRSA DNA—a detection process that’s far faster than traditional culturing processes. The focus is not whether a patient is infected, but the colonization status of the MRSA bacteria. According to Kiechle, about one-third of patients who have colonized MRSA on their person will develop an infection after they undergo surgery. Treatments include a twice daily application of bactroban in the nasal passages, a daily bath or wipedown using chlorhexidine, and delays in undergoing invasive procedures. The process lasts for seven days.
Memorial uses Roche’s MRSA Advanced Assay test, with lab work for all hospitals undertaken at Memorial Regional Hospital South in Hollywood. Positive rates have held fairly steady, from 16.95 percent in October 2008 when the screening program began to just over 15 percent through most of 2012. But the number of MRSA infections plunged. Memorial reported 184 cases systemwide in 2008. In 2012 it reported 24—not only a huge drop from four years prior, but a 67 percent drop from 2011, when there were 72 cases. When the testing program began, the MRSA infection rate within the Memorial system was 14.8 per 1,000 discharges. By 2011, it averaged 1.1 infections per 1,000 patients.
It costs Memorial $25 to perform a single MRSA lab test and $10.49 to perform a decolonization. That compares to Memorial’s average cost of more than $9,400 to treat a MRSA infection. As a result, Memorial’s MRSA prevention program saved more than $362,000 in 2010 alone and an estimated $1.39 million since it was introduced.
The cost-avoidance of preventing MRSA infections since the program was implemented in 2008 ranges from almost $4 million (using $9,449 per infection as reported by Memorial Healthcare System) to $11 million (using $27,000 per MRSA infection as reported in literature).
‘The Bug Stops Here’
John T. Mather Hospital is not an urban multihospital system like Memorial but instead a community hospital of less than 250 beds on Long Island’s North Shore. Nevertheless, it launched an HAI containment program in 2008 that was no less ambitious than Memorial’s. Titled “The Bug Stops Here,” the program’s goal is to reduce the HAI rate to zero.
“It’s an initiative that engaged everyone from the board of directors to the people who clean the rooms,” said Uettwiller-Geiger. Infection control crews make rounds of the hospital floors daily, and hospital management does not hesitate to isolate patients who are at risk for spreading infections—even though Mather only employs semiprivate rooms.
Like Memorial, Mather embraced molecular testing specifically because of the rapid turnaround time, and it chose to bring testing for HAIs in-house. The traditional culturing process requires two days and sometimes up to four days to yield results—more than enough time for an infection to spread among patients. Like Memorial, Mather tests patients via nasal swabs.
“It allows us to return to the physician actionable information in less than an hour,” Uettwiller-Geiger said—a 96 percent faster response time than the traditional testing methods. Moreover, testing for HAIs takes place around the clock, using the Cepheid GeneXpert testing system.
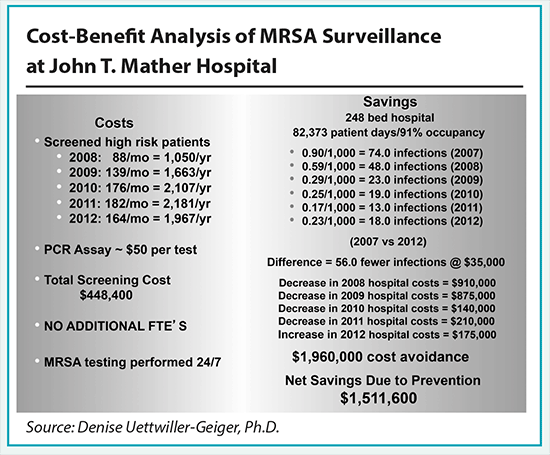
Testing patients suspected of carrying MRSA is pricey compared to Memorial, running about $50 a test, Uettwiller-Geiger said. By 2012, Mather was spending just under $100,000 a year on MRSA testing alone. It has spent about $450,000 in total since the initiative launched.
However, the MRSA infection rate went from 0.9 infections per 1,000 patient days prior to the launch of the initiative to as low as 0.17 per 1,000 in 2011 (it crept back up to 0.23 last year). According to Uettwiller-Geiger, the rise last year represented five additional infections and could be a statistical anomaly.
“We’d like to get to zero, but it may not be realistic,” she conceded.
Nonethless, Uettwiller-Geiger concluded that the testing avoided at least 56 MRSA infections, saving about $35,000 in treatment per case. Altogether, that resulted in avoiding $1.96 million in additional costs, meaning the investment in the additional lab work has saved the hospital $1.51 million to date, or an average of $300,000 per year.
The results were equally impressive in treating C. diff. Mather’s lab used two different tests to determine not only the presence of C. diff. but also whether it is generating life-threatening toxins. The combined cost for the two tests is $52.
Between 2010 and 2012, Mather spent $86,460 testing for C. diff. Its infection rate dropped from 0.95 per 1,000 patient days in 2009 to 0.34 per 1,000 in 2012. Altogether, 44 infections were prevented, resulting in a cost avoidance of $1.54 million and total savings of $1.45 million.
Additionally, the average length of stay in the intensive care and critical care units dropped from 4.4 days in 2007 to 3.3 days in 2012, saving Mather another $491,000 a year.
Nevertheless, Mather remains under the financial pressure faced by many community hospitals these days, prompting it to recently discontinue its defined contribution pension plans and offer buyouts to some employees.
But when Uettwiller-Geiger recently encountered Mather’s chief financial officer in the hospital coffee shop, she learned that she would not be among those tendered a buyout offer.
“I was told that I killed more than I eat,” she said.