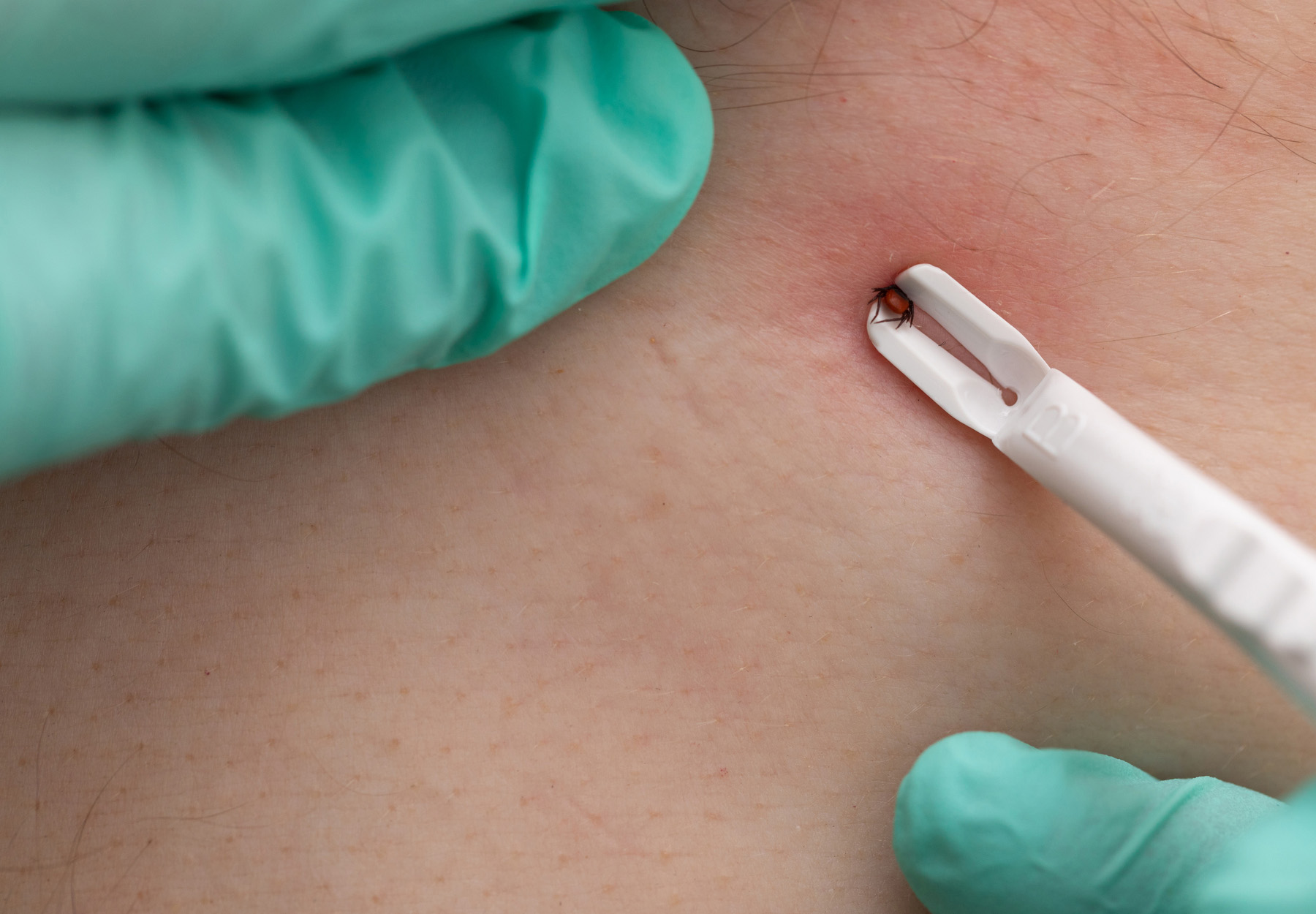
Is an Antibody Test for Early Detection of Lyme Disease Feasible?
Recent study suggests a new test may help doctors detect Lyme disease during the early stages when treatment is likely to be more effective.
Subscribe to Clinical Diagnostics Insider to view
Start a Free Trial for immediate access to this article