July Update of CLIA-Waived Tests, Billing Codes
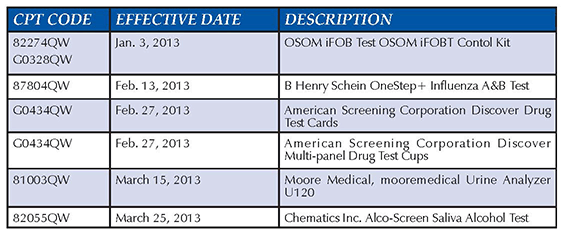
The July 1, 2013, update to the list of tests waived under the Clinical Laboratory Improvement Amendments (CLIA) includes six tests approved by the Food and Drug Administration. When billing for the tests below, you must use the QW modifier. This enables your local Medicare contractor to recognize the code as waived. The July update, plus a complete list of CLIA-waived devices, can be found in Change Request 8301, at http://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/Downloads/R2734CP.pdf. Associated Data
The July 1, 2013, update to the list of tests waived under the Clinical Laboratory Improvement Amendments (CLIA) includes six tests approved by the Food and Drug Administration.
When billing for the tests below, you must use the QW modifier. This enables your local Medicare contractor to recognize the code as waived.
The July update, plus a complete list of CLIA-waived devices, can be found in Change Request 8301, at http://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/Downloads/R2734CP.pdf.
Associated Data
Subscribe to view Essential
Start a Free Trial for immediate access to this article