Medicare Contractors Revise MoPath Pricing
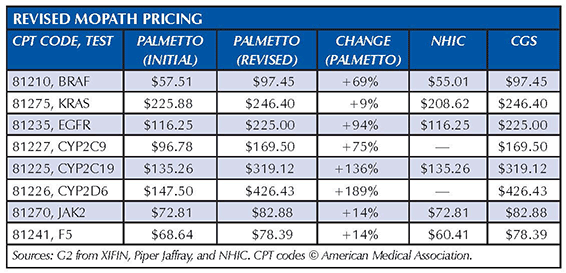
Several Medicare Administrative Contractors (MACs) in the last two weeks have increased pricing for eight molecular pathology codes that industry sources said were initially priced significantly below cost. Palmetto GBA, the MAC for California, Nevada, Hawaii, and the U.S. Pacific territories, was the first to announce that it was increasing pricing for the codes, which include BRAF, KRAS, EGFR, CYP2C9, CYP2C19, CYP2D6, JAK2, and F5. Cigna Government Services and National Heritage Insurance Corp. soon followed. The revised pricing is based largely on information submitted by the California Clinical Laboratory Association, which had argued that the initial MAC payment rates were too low for labs to continue performing some critical molecular diagnostic tests. The pricing increase ranged from 9 percent for CPT code 81275 (KRAS) to 189 percent for CPT 81226 (CYP2D6). National Government Services also updated fees in mid-April, but they matched the original pricing released by Palmetto, according to Kyle Fetter, assistant vice president, molecular diagnostics services, XIFIN Inc., which helped coordinate the lab data submitted to Palmetto. “This would appear to be a timing issue, and we still expect to see Medicare contractors come into line with Palmetto’s new pricing,” he wrote in a blog posting at www.xifin.com. […]
Associated Data
Subscribe to view Essential
Start a Free Trial for immediate access to this article