New Breakthrough Device Designations Fall 37 Percent in 2022
According to the FDA’s recently-released annual report, only 135 devices received the designation last year, versus 213 in 2021.

The COVID-19 pandemic continues to take a toll on the development of new laboratory products that do not target the SARS-CoV-2 virus. According to the newly released 2022 Annual Report from the FDA Center for Devices and Radiological Health (CDRH), only 135 medical devices received breakthrough device designation last year, 37 percent fewer than the 213 breakthrough designations CDRH awarded in 2021. However, the number of breakthrough devices that have received marketing authorization increased from 13 to 19.1
The FDA Breakthrough Devices Program
The Breakthrough Devices Program is a voluntary initiative that enables manufacturers of certain medical devices and device-led combination products to interact with and get feedback from the FDA’s experts on issues that may arise during the premarket review phase. In addition to helping resolve potential bottlenecks in premarket, 510(k), and De Novo review, manufacturers that participate in the program generally get prioritized review for their submissions. The result is quicker clearance and earlier access for patients and providers to novel medical devices and combination products.
Established in 2015 to replace the Expedited Access Pathway (EAP) and Priority Review for medical devices, use of the Breakthrough Devices Program had been steadily growing in each year of its existence. However, after peaking in 2021, designations declined for the first time in 2022.
Number of Granted Breakthrough Device Designations by Fiscal Year*
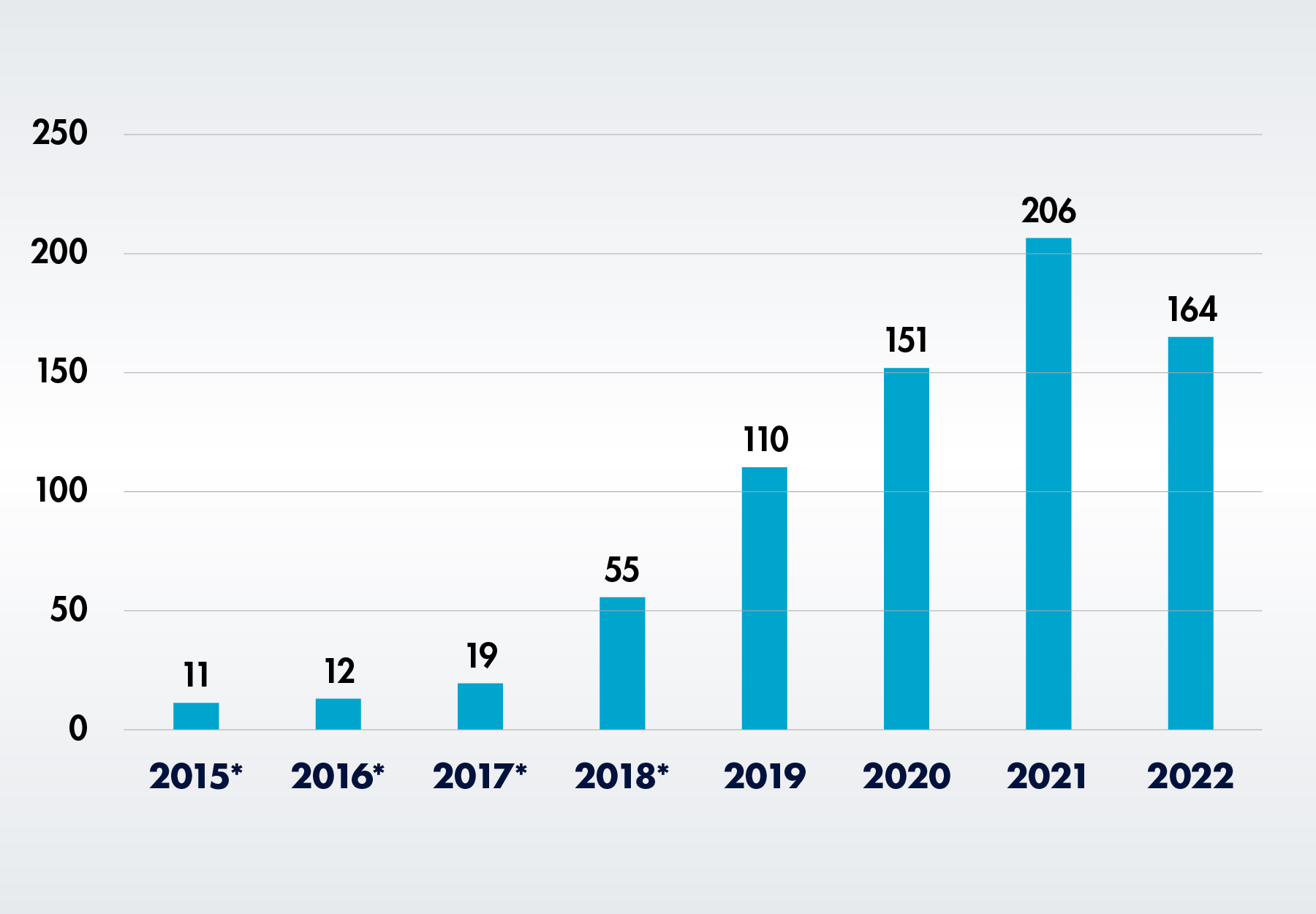
*Numbers include devices originally designated under the EAP, as well as designations not granted by CDRH.
Overall, 752 devices have received breakthrough device designation since the program’s inception, including 728 from CDRH and the Center for Biologics Evaluation and Research (CBER). The report does not offer an explanation for last year’s decline, other than to note in general that 2022 was “another year of heavy workload and strained resources” for the agency. Meanwhile, CDRH says it has “made enormous strides in reducing” the backlog in reviews caused by the pandemic and reiterates that it is shifting its focus to helping companies transition from COVID-19 conditions.
The report of declines in new breakthrough device designations comes at a time when the FDA has been reassessing program criteria. In October 2022, the agency published proposed new draft guidance to incorporate a new factor into its decision about whether to recognize a product as a breakthrough device: healthcare equity and access.3 According to the guidance, for designation purposes, products that are accessible to underserved and underrepresented populations would likely be deemed as providing “more effective treatment or diagnosis” than existing options. (For more, see “Want Breakthrough Device Designation? You’ll Need to Consider Health Equity,” DTET, December 2022).4
References:
****
Here are the key new laboratory testing and diagnostics product clearances that were announced from mid-January to mid-February 2023:
New FDA Approvals & Emergency Use Authorizations (EUAs)
| Manufacturer(s) | Product |
| Guardant Health | Approval of Guardant360 CDx liquid biopsy assay as companion diagnostic to identify individuals with ESR1 mutant breast cancer who may benefit from treatment with Menarini’s Orserdu (elacestrant) |
| Shanghai Genext Medical Technology | 510(k) clearance for Tacrolimus Assay Kit |
| BioMérieux | 510(k) clearance for VITEK 2 AST-Gram Positive Moxifloxacin assay |
| Thermo Fisher Scientific | 510(k) clearance for Sensititre YeastOne Susceptibility System |
| Becton Dickinson | 510(k) clearance for BD Veritor System for Rapid Detection of Flu A+B CLIA-waived kit |
| Qiagen | Premarket approval for use of Sodium Heparin tubes as blood collection alternative with Quantiferon TB Gold Plus Test |
| Micronoma | Breakthrough device designation for OncobiotaLung blood-based test categorizing lung nodules into high or low risk of malignancy |
| Scope Molecular Laboratory | EUA for SARS nCoV-2019 Multiplexed Assay RT-PCR test for serial COVID-19 testing |
| Selux Diagnostics | 510(k) clearance for Next Generation Phenotyping (NGP) System along with test panel to determine antimicrobial susceptibility of gram-positive bacteria |
| Cytovale | 510(k) clearance for IntelliSep test for early detection of sepsis |
| DiaCarta | EUA for QuantiVirus MPXV Test Kit, monkeypox PCR test |
| Prenetics Global | Clearance for ACTOnco, a comprehensive genomic profiling test for solid tumors developed by ACT Genomics |
Global Report
Europe: New Product Approvals in Europe
| Manufacturer(s) | Product(s) |
| Abbott | TactiFlex Sensor Enabled Ablation Catheter for treating arrhythmias such as atrial fibrillation (AFib) |
| Infinity Neuro | Inspira aspiration catheters for treating stroke |
| Geneseeq Technology | Shielding multi-cancer minimal residual disease detection (MRD) kit |
| Geneseeq Technology | MERCURY multi-cancer early detection (MCED) kit |
| MedMira | Vyra CoV2Flu Antigen Test combination assay for SARS-CoV-2 and influenza A and B |
Other international clearances announced during the period:
| Manufacturer(s) | Country(ies) | Product(s) |
| Beijing Genome Precision Technology (Bionano Genomics’ Chinese manufacturing partner) | China | Class I registrations for Bionano’s DNA extraction kit and labeling products |
| Panagene | Korea | OncoTector KRAS mutation test kit approved as grade 3 companion diagnostic to support non-small cell lung cancer (NSCLC) treatment Lumakras (ingredient: sotorasib) |
| Sysmex | Japan | Blood-based HISCL β-Amyloid 1-42 Assay Kit and HISCL β-Amyloid 1-40 Assay Kit for Alzheimer’s disease |
Subscribe to Clinical Diagnostics Insider to view
Start a Free Trial for immediate access to this article