Proposed changes to Medicare’s provider enrollment provisions could have serious consequences for laboratories that mistakenly submit an improper claim for services provided.
In a rule published in the April 29 Federal Register, the Centers for Medicare and Medicaid Services (CMS) proposed changes to the provider enrollment process that include not just the denial of the enrollment application but a revocation of a provider’s or supplier’s ability to bill the Medicare program. All currently enrolled providers could be affected by these changes.
The new provider enrollment provisions would allow denial of a provider’s or supplier’s enrollment if it is the current owner or was the owner of another provider or supplier that had a Medicare debt when the latter’s enrollment was voluntarily or involuntarily terminated or revoked depending on certain criteria. The rule also would allow denial of enrollment or revocation of Medicare billing privileges if the provider, supplier, owner, or managing employee was convicted of a felony within the past 10 years. It would also allow revocation of Medicare billing privileges “if the provider or supplier has a pattern or practice of billing for services that do not meet Medicare requirements.”
Other changes include a requirement that revoked providers and suppliers submit their remaining claims within 60 days of their revocation, limits on the ability of ambulance companies to “back bill” for services furnished prior to enrollment, and elimination of the ability of revoked providers and suppliers to submit a corrective action plan in certain circumstances.
The part of this new rule that could have significant impact on currently enrolled providers and suppliers, like clinical laboratories, is the section concerning abuse of billing privileges. Currently, a provider’s or supplier’s Medicare billing privileges may be revoked if it submits a claim for services that could not have been furnished to a specific individual on the date of service. The rule provides some examples, such as when the beneficiary is deceased, when the directing physician or beneficiary is not in the state or country where the services were furnished, or when the equipment necessary for testing is not present where the testing is said to have occurred.
The rule proposes to expand these revocation reasons to permit revocation if CMS determines that the provider or supplier “has a pattern or practice of billing for services that do not meet Medicare requirements, such as but not limited to, the requirement that the services be reasonable and necessary.” This is different from existing revocation requirements because it addresses overall billing patterns rather than individual claims, and in these cases services were furnished but the claims do not meet Medicare requirements.
According to comments by CMS in the rule, it would “place providers and suppliers on notice that they are under a legal obligation to always submit correct and accurate claims.” A laboratory or other provider can have its Medicare billing privileges revoked if it has, for instance, ineffective billing software that allows a lot of improper claims to be submitted, even if those claims are ultimately denied by Medicare for medical necessity or other reasons. CMS states, “We believe that a provider or supplier should be responsible for submitting valid claims at all times and that the provider or supplier’s repeated failure to do so poses a risk to the Medicare Trust Fund.”
Comments by Providers and Suppliers
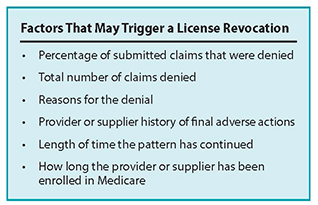
CMS is seeking comments on what constitutes “a pattern or practice” under these changes. The proposed rule provides a list of what might trigger an action (see chart on page 4). CMS wants to receive comments on these factors including whether additional factors should be considered and what those might be, which of the listed factors should not be considered, which of these factors should be given greater weight, if any, and finally, whether there should there be a minimum or maximum threshold established for percentage of claims denied and total number of claims denied.
CMS would also like to know if the provider and supplier community thinks that revocation is only warranted if the provider or supplier submitting the claims does so with knowledge or “reckless disregard” as to meeting Medicare requirements. CMS wants to assure the provider community that these changes are not meant to revoke billing privileges for isolated and sporadic claims denials or for innocent errors in billing. The focus is on situations where a provider or supplier regularly fails to submit accurate claims.
Summary
There are many other important aspects of this rule, and all laboratory compliance officers and billing managers should be aware of its contents. CMS is required to respond to all comments on regulations, and this is certainly a regulation that needs comment. If these regulations go through as written, a provider’s or supplier’s best defense is an effective and active compliance program that includes a regular system of audits and monitors of claim submittal. The emphasis on the monitors should be how many denials there are, what the denials are for, and preventive action to eliminate them to the extent possible. This is important because monitors are performed more frequently than audits, and a laboratory wouldn’t want to establish a pattern or practice of submitting claims that get consistently denied because it didn’t notice until its annual comprehensive billing audit.
Associated Data