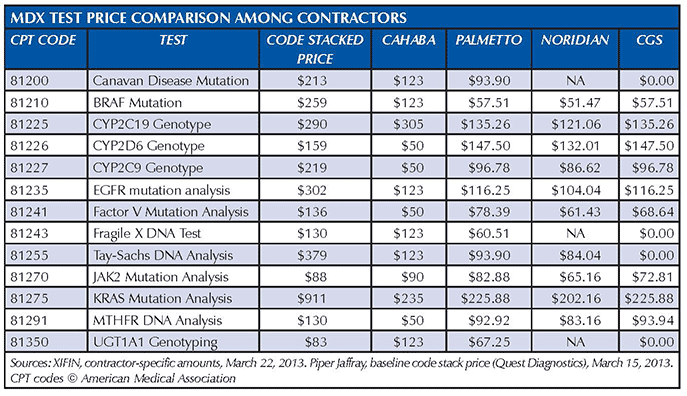
As a result of displeasure with preliminary gap-fill pricing for MDx codes, many providers and their associations are pressuring local Medicare Administrative Contractors (MACs) to revise the payment rates upward, submitting additional cost data to justify such a move.Currently, the contractor-specific rates are set in the range of 20 percent to 30 percent below the median reimbursements provided under the previously used code-stacking method.
At issue are some 92 Tier 1 molecular pathology codes recognized by Medicare this year. The Centers for Medicare and Medicaid Services (CMS) assigned these codes to the Part B clinical lab fee schedule but punted the job of pricing them to its local contractors via the gap-fill method that relies on local pricing patterns. CMS said it lacked sufficient information to establish national payment rates for these codes in 2013, despite objections from industry stakeholders who contended they had furnished the agency with enough data on which it could act.
In comments to
NIR on the gap-fill trends, Lale White, executive chairman and CEO of San Diego-based XIFIN, a revenue cycle management company for diagnostic service providers, noted, “The prices released to date clearly exemplify the lack of understanding of not just the economics of performing complex MDx services, but also of their value in reducing overall health care spending while improving outcomes.
“While all MACs were instructed to perform a thoughtful gap-fill pricing exercise, many are simply replicating the flawed results of others with some minor tweaks. Private payers appear to be following suit without thoughtful review and consideration of the reasonableness of established rates.
An analysis by equity research firm Piper Jaffray speculates that given grassroots and political pressures on the MACs, the overall rates for 2014 will be below the company’s baseline Quest Diagnostics code stacks, closer to 10 percent rather than the 20 percent to 25 percent median cuts that it has tracked thus far.
“The paradigm shift in health care and promise of personalized medicine could be cut off at the knees by short-term thinking ascribing greater importance to saving a few pennies on the front end than recovering millions on the back. All lab tests eventually become commoditized, but not prior to market acceptance followed by automation breakthroughs. It appears that economic and political expediency may be trumping a more common sense approach. Some of the highest volume molecular diagnostic tests have seen a fee reduction of 70 percent to 90 percent compared with their reimbursement under stacked coding.
“This leaves labs and manufacturers in the uncomfortable position of having to either remove a high-value service from their menu or risk going out of business. While the industry is in high gear fighting for its life, we are hopeful that MACs and decision makers at the federal level will provide some relief before it is too late for many fine research labs.”
In response to industry reaction about the gap-fill fees, Palmetto, the MAC for California, Nevada, Hawaii, and U.S. Pacific territories, has agreed to review information and evidence supporting a change. It has requested that labs get together to aggregate their data and provide it all in one set, and the California Clinical Laboratory Association (CCLA) has requested that XIFIN serve as the point of collection and consolidator of the information. CCLA encourages nonmembers to participate in order to provide a more robust data set, XIFIN said in a statement, and “we are aggregating data for both CCLA members and non-CCLA labs.”
For more on pricing of molecular pathology codes, see G2 Intelligence’s newest report: Medicare’s New Payment System for Molecular Tests: Coding Methodology, Reimbursement Strategies, Rate Updates,
available
in early April. Details available at www.G2Intelligence.com/MDxPaymentGuide.
Timetable for Gap-Fill Rates and Public Comment
CMS has given its MACs until April 1 to complete gap-fill pricing for the molecular pathology codes new to the Part B lab fee schedule. The agency will post the interim contractor-specific rates on its Web site by April 30 for a 60-day period of public comment (not reconsideration requests).
When CMS finalizes the rates in August or September, it will accept reconsideration requests on the gap-fill amounts for 30 days. Once the reconsideration process is completed, CMS will publish the final national fee caps for these codes in the 2014 lab fee schedule (typically released in November or December) and they will not be subject to further reconsideration.