Roche Scores First European IVDR Approval for Diagnostic Tests
The test manufacturer is the first to gain IVDR certification for a companion diagnostic under the new regulation.
Despite vigorous protest from the diagnostics industry, notified bodies, and other stakeholders, the European Union (EU) stuck with its decision to put the In Vitro Diagnostic Regulation (IVDR) into effect on May 26, 2022.1 The unsurprising result of this ready-or-not approach to implementation has been much slower than the anticipated volume of new IVDR approval applications. However, one company that is not backing off is Roche. Last summer, Roche Diagnostics became the first manufacturer to win IVDR certification for a Class D product. Now Roche has replicated the feat by securing the first certification for a companion diagnostic under the new regulation.
You Don’t Have to Be in Europe to Be Covered by IVDR
The New IVDR System
EU regulation of in vitro diagnostic medical devices has existed since 1993. However, the IVDR requirements are far more comprehensive and stringent than those contained in its predecessor regulation, the so-called In Vitro Diagnostic Directive (IVDD), aka Directive 98/79/EC.2 The term “medical device” covered by the new regulation is defined broadly as encompassing any device used to examine or monitor humans, including not just diagnostic assays for infectious diseases or genetic conditions but also just about everything used in the laboratory testing process, from specimen receptacles to results analysis software.
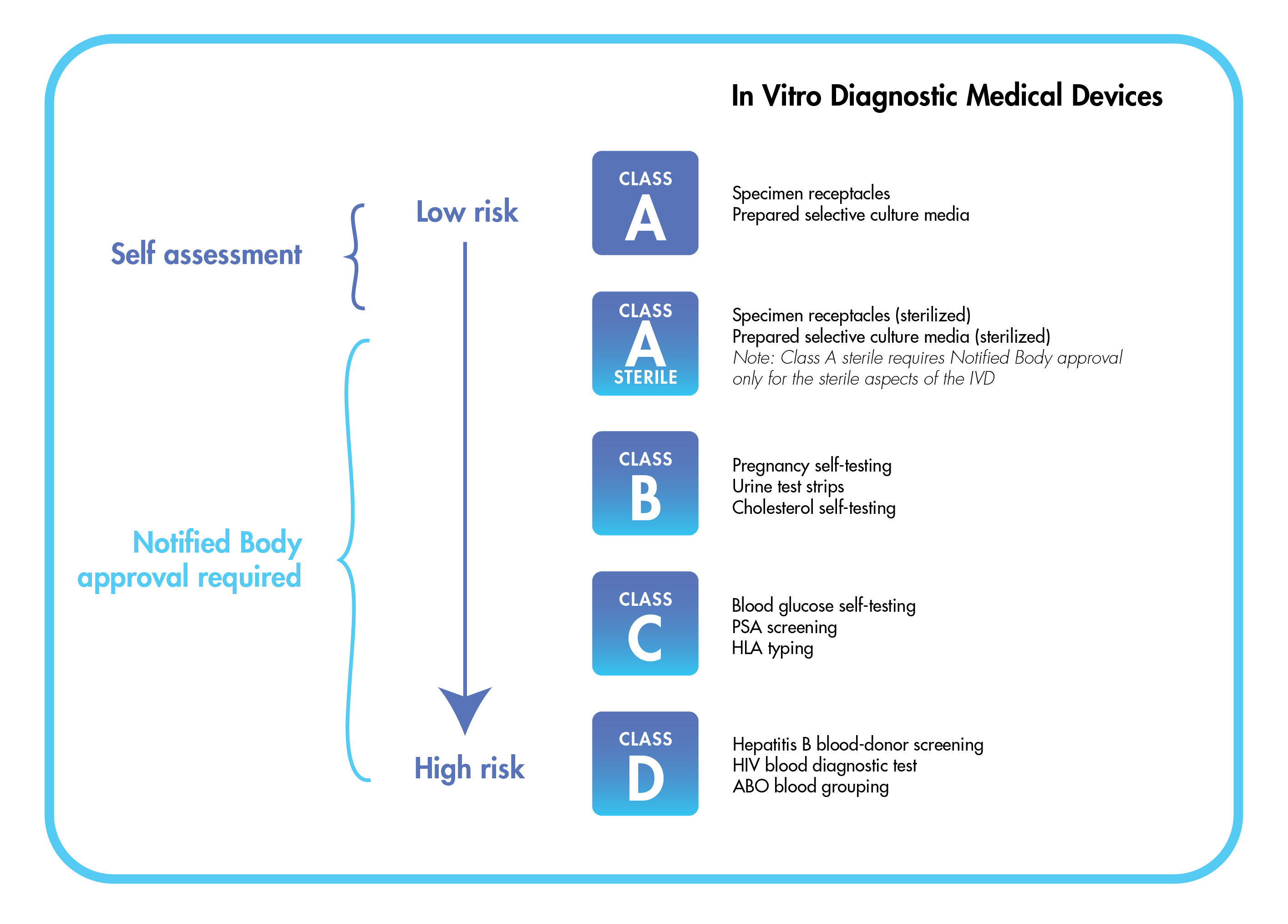
Perhaps the most significant difference between the IVDR and predecessor IVDD regime is that most covered products cannot be marketed unless and until they are assessed and cleared by EU-approved entities known as “notified bodies” (NBs). Under previous rules, it was possible for companies to obtain a CE marking via the use of a self-certification process. The requirements that a product must meet to garner certification vary depending on its risk classification. There are four categories, A to D, with A representing the lowest risk and D the highest. Class A IVD products can be self-certified; all other device classes require NB conformity assessment and approval.
IVDR Device Risk Classifications
The reason IVDR applications have come at a trickle rather than a flow is not just how burdensome the new requirements are. There is also a shortage of NBs available that can perform the required conformity assessment, with the EU having designated only seven such entities so far. They include:
- BSI Group The Netherlands B.V. (Netherlands)
- DEKRA Certification B.V. (Netherlands)
- DEKRA Certification GmbH (Germany)
- GMED SAS (France)
- TÜV Rheinland LGA Products GmbH (Germany)
- TÜV SÜD Product Service GmbH (Germany)
- 3EC International a.s. (Slovakia)
Roche Diagnostic Products Make IVDR Certification History
So far, Swiss giant Roche has blazed the trail for new product certification. Last July, TÜV SÜD Product Service officially certified a qualitative immunoassay detecting SARS-CoV-2 antibodies in human blood plasma and serum from Roche, making it the first Class D product to receive IVDR certification.
“The certificate now issued marks the first time that this procedure has been brought to successful completion and represents an important milestone in IVDR implementation,” noted Andreas Stange, VP of medical and health services at TÜV SÜD Product Service, in the announcement.4
But Roche was not finished making history. In December 2022, that same NB, TÜV SÜD Product Service, announced that Roche had received the first IVDR certificate for a Class C product for its qualitative immunohistochemical cancer biomarker assay that detects expression of PD-L1 for use as a companion diagnostic to identify cancer patients that may benefit from immune checkpoint inhibitors. Such treatments include Merck & Co.’s Keytruda and Roche’s own Tecentriq.5 After Class D, Class C is the second highest risk designation in the IVDR system.
References:
- https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0746
- https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:31998L0079
- https://www.clinicallab.com/ivdr-the-eus-in-vitro-diagnostic-regulation-for-medical-diagnostic-tests-25051
- https://www.tuvsud.com/en/press-and-media/2022/july/tuev-sued-issues-first-ivdr-certificate-for-a-class-d-medical-device
- https://www.tuvsud.com/en-us/press-and-media/2022/december/tuev-sued-issues-the-first-ivdr-certificate-for-an-ivd-companion-diagnostic-device
****
Here are the other key new laboratory testing and diagnostics product clearances that were announced from mid-December 2022 to mid-January 2023:
New FDA Approvals & Emergency Use Authorizations (EUAs)
| Manufacturer(s) | Product |
| Precision BioLogic | 510(k) clearance for CRYOcheck Chromogenic Factor IX test for managing hemophilia B |
| Burning Rock | Breakthrough device clearance for OverC Multi-Cancer Detection Blood Test (MCDBT) for early detection of esophageal, liver, lung, ovarian, and pancreatic cancers in average-risk patients ages 50 to 75 |
| Datar Cancer Genetics | Breakthrough device clearance for TriNetra-Glio liquid biopsy test to diagnose brain tumors inaccessible to conventional biopsy |
| Becton Dickinson | EUA for Viasure Monkeypox virus Real-Time PCR Reagents test for use on BD Max System |
| Visby Medical | EUA for RT-PCR assay to simultaneously detect and differentiate between SARS-CoV-2, influenza A, and influenza B viral RNA |
| Advin Biotech | EUA for Advin COVID-19 Antigen Test @Home for over-the-counter use |
| Oceanit Foundry | EUA for Assure-100 Rapid COVID-19 Home Test antigen assay for over-the-counter use |
| OnsiteGene | EUA for Hi-Sense COVID-19 Molecular Testing Kit 1.0, an RT-qPCR-based assay |
| CTK Biotech | EUA for ImmuView COVID-19 Antigen Home Test, an over-the-counter test |
| Foundation Medicine (Roche subsidiary) | Clearance for FoundationOne Liquid CDx test as a companion diagnostic for Genentech's Rozlytrek (entrectinib) |
| Foundation Medicine | Clearance for FoundationOne Liquid CDx test as companion diagnostic for epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI) |
| MedCognetics | 510(k) clearance for QmTriage AI platform |
| Acon Laboratories | EUA for Flowflex COVID-19 Antigen Rapid Test, nasal swab-based point-of-care assay |
| Yale School of Public Health's Department of Epidemiology of Microbial Diseases | EUA for SalivaDirect COVID-19 assay allowing authorized labs to use pre-assembled SalivaDirect Unsupervised Collection Kits for at-home sample collection |
| Thermo Fisher Scientific | EUA for Applied Biosystems TaqPath Monkeypox/Orthopox Virus DNA Kit, PCR test |
| Thermo Fisher Scientific | De Novo clearance for SeCore CDx HLA Sequencing System as a companion diagnostic to Immunocore’s Kimmtrak (tebentafusp-tebn) therapy for uveal melanoma |
| Agilent Technologies | Clearance for Agilent Resolution ctDx FIRST next-generation sequencing-based liquid biopsy assay as a companion diagnostic to identify patients likely to benefit from Mirati Therapeutics' drug Krazati (adagrasib) for adults with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer |
| Qiagen | Clearance for therascreen KRAS RGQ PCR kit as companion diagnostic to identify patients likely to benefit from Mirati Therapeutics' drug Krazati (adagrasib) for adults with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer |
Global Report
New Product Approvals in Europe
| Manufacturer(s) | Product(s) |
| Roche | In Vitro Diagnostic Regulation (IVDR) certificate for qualitative immunohistochemical cancer biomarker assay that detects expression of PD-L1 for use as a companion diagnostic to assess likely response of cancer patients to Merck & Co.’s Keytruda, Roche’s Tecentriq, and other immune checkpoint inhibitors |
| Virax Biolabs | HPV test kit covering 18 human papillomavirus genotypes |
Other international clearances announced during the period:
| Manufacturer(s) | Country(ies) | Product(s) |
| Sysmex | Japan | Blood-based HISCL β-Amyloid 1-42 Assay Kit and HISCL β-Amyloid 1-40 Assay Kit for Alzheimer’s disease |
| QuidelOrtho | Canada | TriageTrue High-Sensitivity Troponin I test, a single-use fluorescence immunoassay for rapid point-of-care myocardial infarction diagnosis |
| QuantuMDx | UK | Q-POC SARS-CoV-2, Flu A/B & RSV Assay |
Subscribe to Clinical Diagnostics Insider to view
Start a Free Trial for immediate access to this article