FDA: EUAs for COVID-19 Lab Tests Will Continue after PHE Ends
Agency says it will continue to have authority to clear new lab tests and other COVID-19 products on an emergency basis even after the PHE ends.
Agency says it will continue to have authority to clear new lab tests and other COVID-19 products on an emergency basis even after the PHE ends.
As we move further into 2023, COVID-19, add-on, and genetic tests will likely remain targets for federal regulators.
Recent fraud case involving phlebotomy company shows why labs need to pay special attention to Medicare specimen collection and travel allowance rules.
A look at the US end-of-emergency game plan and how it will impact lab compliance.
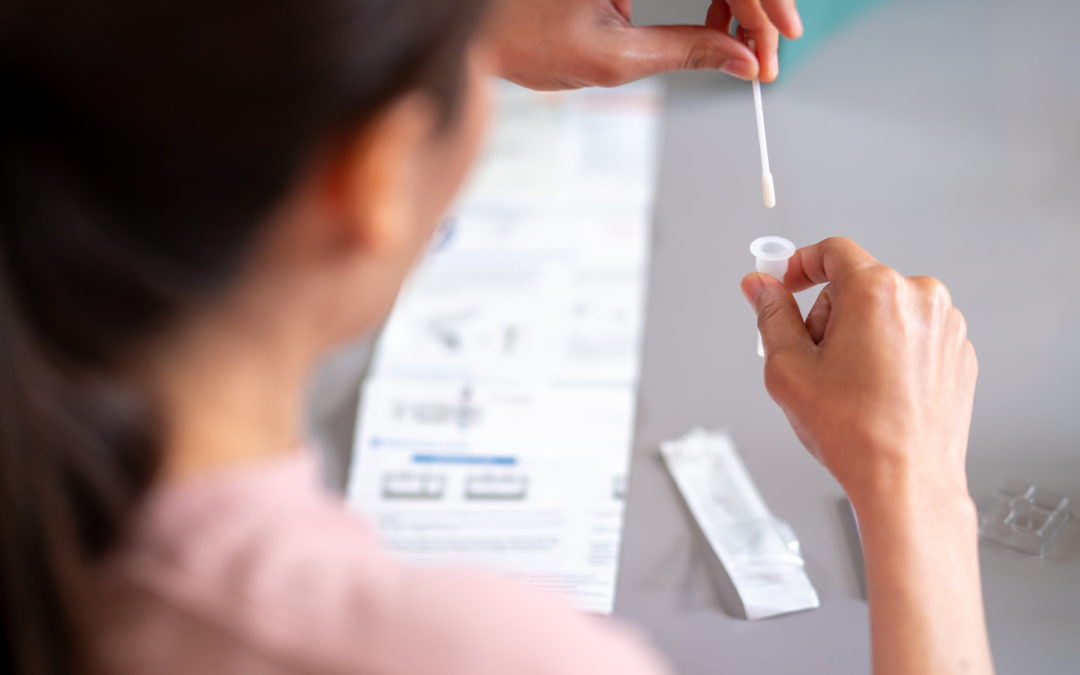
FDA will still be able to issue EUAs for COVID-19 tests after May 11.