
Why Revenue Cycle Management Systems Fall Behind—and How to Modernize Them
Clinical lab billing teams face an overwhelming number of challenges if revenue cycle strategies fall short

Clinical lab billing teams face an overwhelming number of challenges if revenue cycle strategies fall short
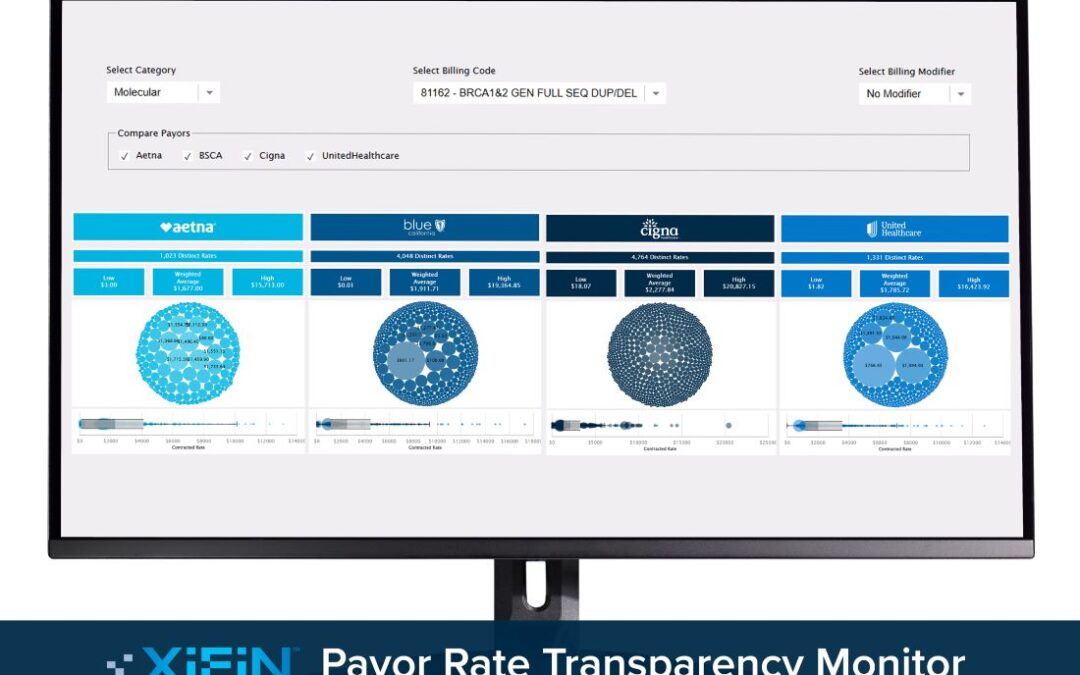
The free online tool can set a foundation for renewed reimbursement strategies and contract negotiations with insurance carriers

On-demand testing doesn’t require registration, billing, or scheduling, keeping costs low while collecting “real” dollars upfront

ACLA president Susan Van Meter discusses some of the key issues labs faced in 2024, along with work to address these developments through 2025

Sara Pirzadeh-Miller offers advice on overcoming the reimbursement barriers genetic counselors face in the clinical lab and beyond