Decoding Quality Terminology
There is a lot of talk about quality these days, but there is often confusion around quality terminology

Editor’s Note: G2 Compliance is pleased to announce our upcoming webinar, The Cost of Poor Quality, with Jennifer Dawson. Register Here
Quality has been defined as fitness for use, conformance to requirements, the pursuit of excellence and a product or service free of deficiencies1. There is a lot of talk about quality these days, as there should be, but I find there is confusion around quality terminology. I hear quality assurance and quality control used interchangeably.
If you run a Google search for the terms “quality assurance” and” quality control”, I am 99% certain your search will return a number of differing definitions. “Quality management”, “Total quality management” and “quality improvement” are additional terms that are used frequently, adding to the confusion. Many of these terms are often used interchangeably. You may think these terms are more alike than different as they all contain the word “quality”, but they are not synonymous.
Let’s explore some definitions to make sense of this alphabet soup.
Quality Control (QC)
- Procedures used in each assay to assure a test run is valid and results are reliable1
- A system for verifying and maintaining a desired level of quality in an individual test or process3
- A generic term that refers to the monitoring and assessment of laboratory testing processes to identify problems and maintain performance26
- The operational techniques and activities used to fulfill requirements for quality8
Quality Assurance (QA)
- A part of quality management focused on providing confidence that quality requirements will be fulfilled7
- A formal and systematic exercise in identifying problems in medical care delivery, designing activities to overcome the problems, and carrying out follow-up monitoring to ensure that no new problems have been introduced and that corrective steps have been effective11
- A broad spectrum of evaluation activities aimed at ensuring compliance with minimum quality standards17
- All actions taken to establish, protect, and improve the quality of health care12
NOTE: The author of this definition, Avedis Donabedian, opined that one cannot guarantee or assure quality. Instead, he believed the goal was to increase the probability that the health care delivered would be good or better quality and suggested the term continuous quality improvement.12
Quality Improvement (QI)
- A formal approach to the analysis of performance and systematic efforts to improve it4
- Systematic and continuous actions that lead to measurable improvement in health care services and the health status of targeted patient groups8
- Defining standards of care, reassessing those standards periodically, and continuously improving the medical systems that support those standards10
- A set of techniques for continuous study and improvement of the processes of delivering health care services and products to meet the needs and expectations of the customers of those services and products. It has three basic elements: customer knowledge, a focus on processes of health care delivery, and statistical approaches that aim to reduce variations in those processes11
Quality Management (QM)
- The application of a quality management system in managing a process to achieve maximum customer satisfaction at the lowest overall cost to the organization while continuing to improve the process8
- Management activities and functions involved in determination of quality policy and its implementation through means such as quality planning and quality assurance (including quality control)14
- Quality management is the act of overseeing all activities and tasks needed to maintain a desired level of excellence. This includes the determination of a quality policy, creating and implementing quality planning and assurance, and quality control and quality improvement15
- All activities of the overall management function that determine quality policy objectives and responsibilities; and implement them by means such as quality planning, quality processes, quality control, quality assessment, and quality improvement within the quality system26
Quality Management System (QMS)
- Management system to direct and control an organization with regard to quality5,7
- A formalized system that documents the structure, responsibilities and procedures required to achieve effective quality management. A QMS helps coordinate and direct an organization’s activities to meet customer and regulatory requirements and improve its effectiveness and efficiency on a continuous basis8
- The organizational resources, processes and procedures to implement quality management, which is broader than both quality assurance (QA) and quality control (QC). Besides QA, the laboratory quality management system also includes management of equipment, supplies and inventories, management of capital, finances and budgeting, and providing training and continuous support of staff and customer service16
- The organizational structure, resources, processes, and procedures needed to implement quality management26
Total Quality Management (TQM)
- A management approach to long-term success through customer satisfaction8
- A management philosophy that seeks to integrate all organizational functions (finance, production, customer service, etc.) to focus on meeting customer needs and organizational objectives13
- A business philosophy that the long-term success of a company comes from customer satisfaction. TQM requires that all stakeholders in a business work together to improve processes, products, services and the culture of the company itself15
As laboratory scientists, I think we can all agree with the definition of QC. Historically, we have been very good at controlling quality in the analytical phase and we know what this entails. The definition of QA however is surrounded by a little more controversy within the lab community, healthcare as a whole and amongst quality professionals working in different sectors. Healthcare source include description of QA as being reactive, retrospective, policing, and in many ways punitive4. That it often involves determining who is at fault after something went wrong and focuses on finding the culprit. This term is also described as being outdated4,10. I’d like to propose that as an industry we adopt the definition that QA is ensuring compliance against necessary standards. This includes QC and regulatory requirements such as CLIA, CAP and TJC. In contrast to QA, the definitions for QI, QM, QMS and TQM are fairly consistent and seem to be accepted even across industries.
Tying it all together
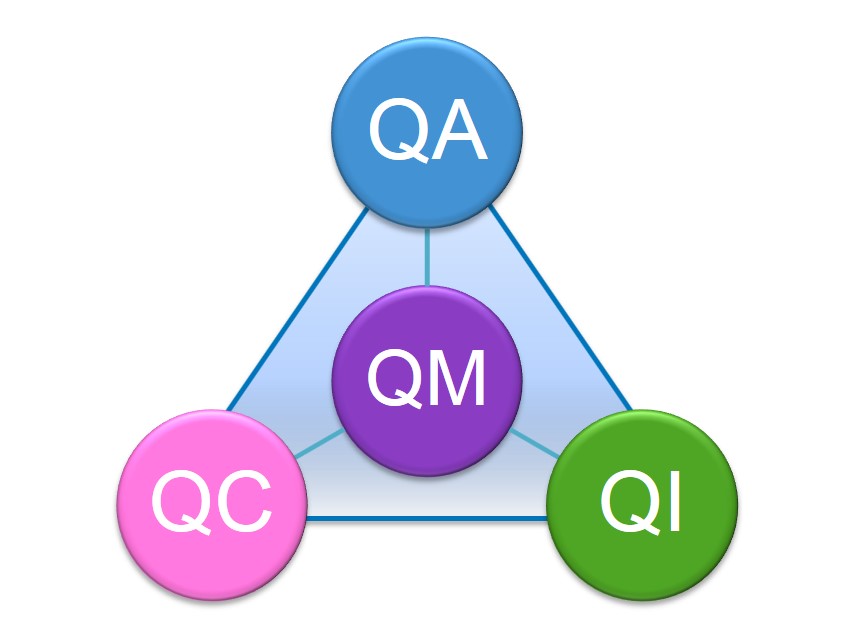
Now that we’ve defined the terms, let’s examine how they relate to each other. If you examine the definitions, it appears that the terms build on each other and increase in scope. It is widely accepted by quality professionals, particularly those in other industries, that the progression of quality started with QC, followed by QA, QM and finally TQM5,18-25. It is also accepted that as you go up the progression, each new phase includes the previous (figure 1). Applying my preferred definition of QM that it is quality planning, assurance, control and improvement and think of QM as an equation, the result is QM = QC + QA + QI (figure 2), where QM includes a QMS. TQM is one step beyond QM where customer, namely patient, satisfaction is the focus.
Figure 1: Hierarchy of Quality Concepts
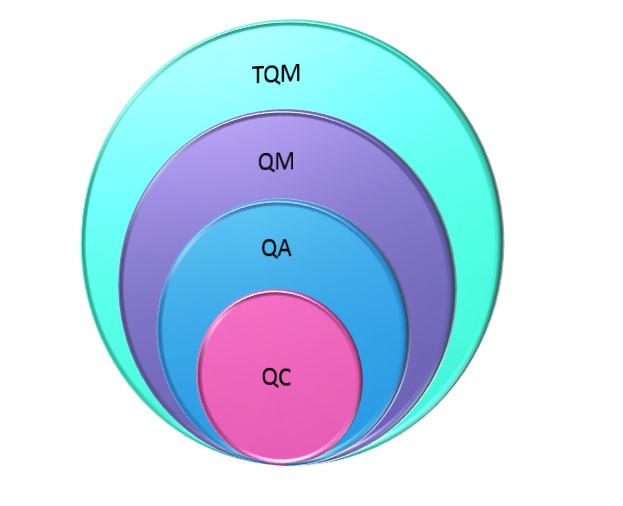
Figure 2: QM = QC + QA + QI
Conclusion
I remember back to when I was working on the bench. I would hear these terms and had no idea what they meant, just that they were related to quality. Had it not been for my career turning in the direction of quality management, I would still not have a solid understanding of their meanings. It is my sincere hope that the information presented in this article provides some clarity in decoding these often utilized and often confused quality terms and buzzwords.
Reference List
- http://asq.org/learn-about-quality/quality-assurance-quality-control/overview/overview.html
- http://www.who.int/diagnostics_laboratory/quality/en/
- https://www.cytopathology.org/quality-control-and-quality-assurance-practices/
- Patient Safety – Quality Improvement Training. Duke University School of Medicine. http://patientsafetyed.duhs.duke.edu/module_a/introduction/introduction.html
- Quality Management System: A Model for Laboratory Services; Approved Guideline – Fourth Edition. CLSI document QMS01-A4. Wayne, PA: Clinical and Laboratory Standards Institute; 2011.
- Perigo D, Rabelo R. ISO-1: Basics on ISO Standards. https://www.westgard.com/iso1.htm
- ISO 9000:2015 – Quality Management Systems – Fundamentals & vocabulary. Geneva, Switzerland. International Organization for Standardization; 2015,
- Quality glossary. https://asq.org/quality-resources/quality-glossary/
- Health Resources and Services Administration. Quality Improvement. https://www.hrsa.gov/quality/toolbox/methodology/qualityimprovement/
- Kunkel M. Quality Assurance and outcomes in outpatient parenteral antibiotic therapy. Infectious disease clinics of North America. Volume 12, Issue 4, 1 December 1998, Pages 1023–1034
- Medicare: A Strategy for Quality Assurance, Volume I (1990) https://www.nap.edu/read/1547/chapter/8
- Healthcare Outcomes Management. Donabedian’s Principles of Quality Improvement. Dale J. Block. Jones & Bartlett. 2006. Pg 10
- Hashmi K. Introduction and Implementation of Total Quality Management (TQM). https://www.isixsigma.com/methodology/total-quality-management-tqm/introduction-and-implementation-total-quality-management-tqm/
- http://www.businessdictionary.com/definition/quality-management.html
- http://www.investopedia.com/terms/q/quality-management.asp
- Dai S. What defines a laboratory quality system? Food Safety Magazine. October 2013. http://www.foodsafetymagazine.com/magazine-archive1/octobernovember-2013/what-defines-a-laboratory-quality-system/
- Health Resources and Services Administration. Developing & Implementing a QI Plan. https://www.hrsa.gov/quality/toolbox/methodology/developingandimplementingaqiplan/part4.html
- QA vs QC – what’s the difference? http://www.iso9001consultant.com.au/QA.html
- Antara P, Shreeti M. OHSAS – A complete description, March 2015. http://www.slideshare.net/AntaraPaul324/ohsas-a-complete-description
- Chaudhary P. Total Quality Management in Healthcare Organizations – An Overview. March 2014 http://www.slideshare.net/poonamchaudhary1/total-quality-management-in-healthcare-organisations
- Chang S. From Good Laboratory Practice to Total Quality Management in Medical Laboratories : The Perspective. October 2002. http://www.slideserve.com/ryo/from-good-laboratory-practice-to-total-quality-management-in-medical-laboratories-the-perspective
- Quality Management Initiatives. https://www.chiyoda-corp.com/csr/en/okyakusama/hinshitsu.html
- Historical development of TQM https://sites.google.com/a/qualindustri.com/qual/quality/history
- QA and QC. http://www.mapwright.com.au/QA_QC.html
- Dawson J. The Lab Quality Continuum. Medical Laboratory Management Magazine. July 2016. https://www.medlabmag.com/article/1298
- Westgard Web. Glossary of QC Terms. https://www.westgard.com/glossary.htm
| Editor’s picks: | ||
From – G2 Compliance Advisor
|
From – National Intelligence Report
|
From – G2 Blog
|
Subscribe to Clinical Diagnostics Insider to view
Start a Free Trial for immediate access to this article