Keep Informed on Legal and Compliance Developments Affecting Diagnostic Labs
Best practices and expert insight to help lab professionals comply with regulations and identify strategic trends in business and technology
Already a Subscriber? Log in Here
Lab Industry Advisor Subscription
Struggling with Staying Informed? In the fast-paced world of diagnostic medicine, staying updated with the latest legal, compliance, regulatory, and industry insights is crucial. Yet, finding reliable and comprehensive information can be overwhelming and time-consuming.
Lab Industry Advisor to the Rescue! Lab Industry Advisor provides comprehensive, up-to-date insights and analyses tailored to your needs. With expert commentary and actionable advice, you’ll stay ahead of the curve. No more sifting through endless sources—everything you need is right here!
Start your free trial today and see how Lab Industry Advisor can transform your approach. Get instant access to invaluable resources designed to keep you informed and competitive. Explore detailed reports, in-depth articles, and exclusive industry insights that help you make better decisions faster. Choose from three subscription levels tailored to fit your needs: Essential, Premium, and Elite.






Additional Business Intelligence Offerings
Lab Industry Advisor Subscriptions Include

Analysis and insight on key compliance, legal, and business developments affecting laboratories
- Updates on changes to regulations
- Healthcare-related laws
- FDA approvals
- Business deals in the diagnostics space, and more
Latest Articles
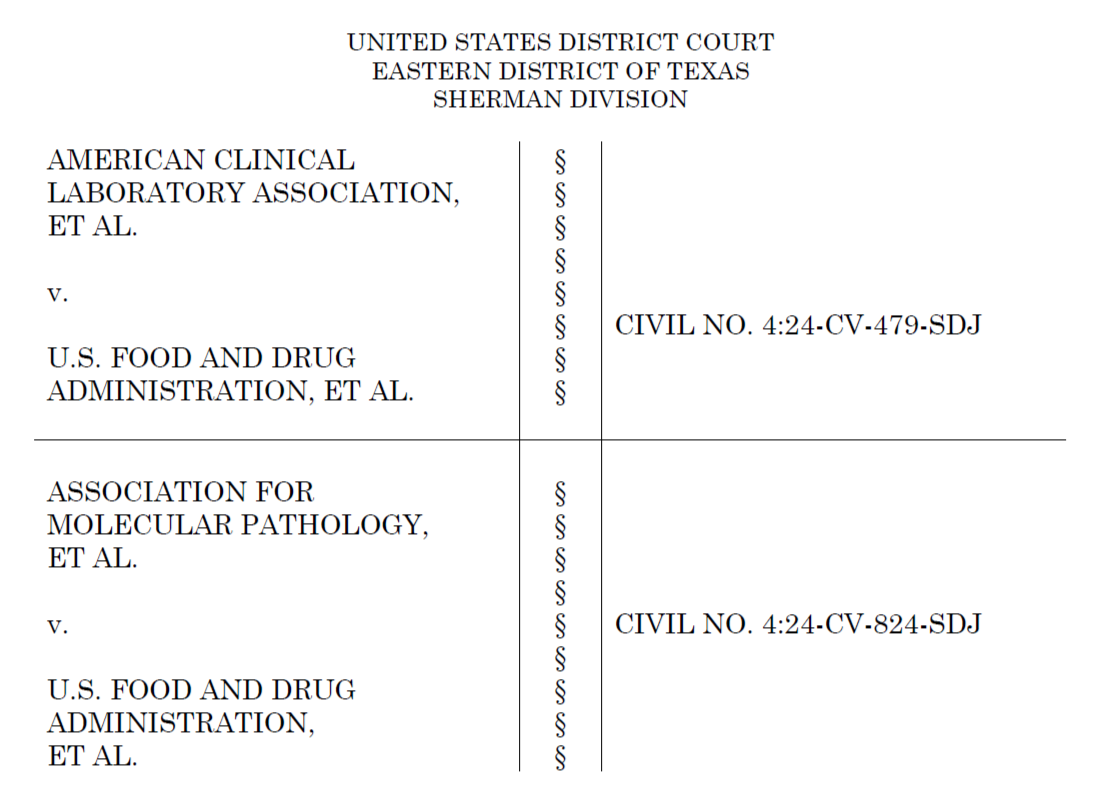
LDTs Rule Update: More Support for Legal Challenges Against FDA
Key clinical laboratory organizations file amicus brief, reiterating that the FDA is overstepping its authority, and its new rule would cause “irreparable patient harm”
Interconnected Clinical Lab Devices Can Increase Operational Capabilities
However, integrated technologies must take into account data protection concerns
LDT Appeal: Looking at the Legal Nuances Behind Potential FDA Actions Following Court Decision
Marginal LDT scenarios could be where the FDA focuses on next, a lawyer notes
The Role of Advanced Molecular Diagnostics in Pathogen Detection
Explore how molecular diagnostics transform pathogen detection, providing faster, more accurate results compared to traditional testing methods
How Advances in T-Cell Receptor Profiling Can Boost Early Cancer Diagnosis
New developments in flow cytometry and molecular biology promise to improve the detection of rare cancers
Diagnostics Pipeline: Savara Introduces Blood Test to Detect aPAP, a Rare Lung Disorder
Savara’s ClearPath assay needs just a few drops of blood to test for aPAP, a condition that can be difficult and invasive to diagnose
Success of Digital Pathology May Come Through its Ability to Handle Volumes
Digital and computational pathology expert Donal O’Shea explores the role of emerging technologies in addressing workforce shortages
Check Out These 5 Tips to Better Retain Clinical Lab Staff
Options include establishing career trajectory plans and offering smooth onboarding for incoming laboratory scientists
LDT Oversight Remains Under CLIA, for Now
Judge rules that the FDA has ‘no authority’ to issue its rule governing laboratory-developed tests
Routine Mass Spectrometry in Clinical Labs: A Competitive Advantage?
How a new automated system bringing MS to routine lab testing could help set laboratories apart from their competitors
FDA Watch: DOGE Changes at FDA Upend Approval Processes, Observers Say
Pre-submission and Q-Submission meetings apparently have been halted, for now