
SCOTUS Won’t Weigh in on How Whistleblowers Make Valid FCA Claims
The court recently denied the petition to hear a case that would have helped offer clarity on what evidence whistleblowers need to file qui tam lawsuits under the FCA.

The court recently denied the petition to hear a case that would have helped offer clarity on what evidence whistleblowers need to file qui tam lawsuits under the FCA.

How to create a written policy banning retaliation for whistleblowing and for investigating retaliation complaints.

What happens when a compliance officer is the whistleblower in a qui tam retaliation lawsuit?

This month’s key lab cases involve false billing of urine drug and PGx tests and two lawsuits that were tossed.
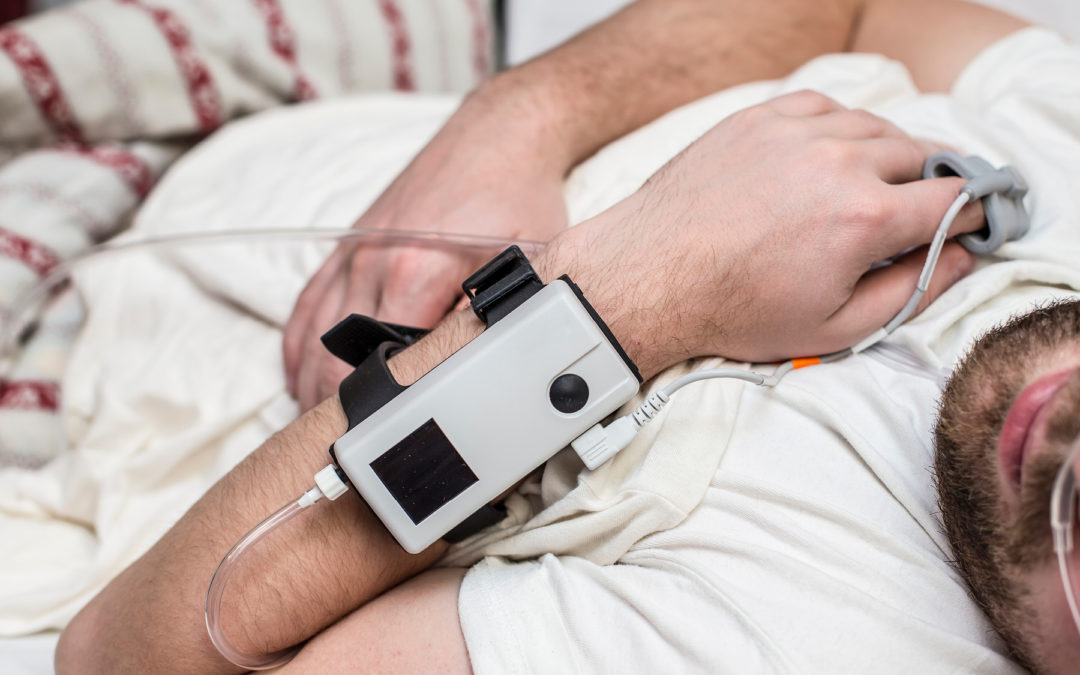
A recent $3.5 million settlement illustrates the kinds of pitfalls that can result in liability for hospital and freestanding sleep labs.