The Benefits of Group A Streptococcus NAAT
Labs that invested heavily in POC molecular tests during COVID-19 are well positioned to leverage this equipment toward GAS diagnostics
As longtime users of a rapid antigen diagnostic test for clinic-based Group A Streptococcus (GAS) pharyngitis, our health system contains a wealth of data available to assess real-world performance. In the wake of the very active 2023 pharyngitis season, a 20-year high by Centers for Disease Control and Prevention estimates, we performed an examination of our internal workflow and performance data.1 The primary concern of our health system was the high number of false negative tests occurring during the active season, especially amongst pediatric patients.
A deep dive into our health systems’ current GAS diagnostic workflows revealed several insights into the benefits that could be gained by changing our practice. Specifically, moving nucleic acid amplification tests into the clinic laboratory setting stands to benefit the patient, provider, and our laboratory teams.
Originating first in research labs, nucleic acid amplification testing (NAAT) has progressed ever closer to the patient. Though many health systems keep the molecular disciplines in a central laboratory, this paradigm is changing.
Rapid antigen diagnostic tests (RADTs)
Most laboratorians are familiar with the benefits of a rapid antigen diagnostic test (RADT), achieving low cost and delivering rapid results. Notably, low sensitivity of an RADT creates the need for additional confirmatory testing, exacerbating the current issues of labor demands and delayed treatment practices.2
In the right setting, RADTs offer a rapid and low-cost method to generate a result. Results of an RADT are considerably more reliable when combined with pre-test probabilities. When using an RADT to rule out COVID-19 on a patient with no symptoms, for example, the negative predictive value exceeds 95 percent.3,4
Why target Group A Streptococcus?
Pharyngitis is predominantly viral in etiology, but in the case of bacterial pharyngitis, GAS reigns supreme as the most common bacterial cause of pharyngitis. If left untreated, GAS can progress to more severe diseases, causing complications such as peritonsillar abscess, cervical lymphadenitis, acute rheumatic fever, post-streptococcal glomerulonephritis, and others.5
When RADTs for GAS are negative, culture confirmation is required by the manufacturer, in alignment with Infectious Diseases Society of America guidelines.6 Bacterial culture requires microbiology expertise; therefore, specimens are transported to a central laboratory for testing. Transport additionally poses associated risks of deterioration of viable target organisms or specimens lost in transit.
Given the risks of associated morbidity, antibiotics may be initiated while final diagnosis is pending. One study has shown up to 30 percent of patients may already be started on an unnecessary antibiotic while awaiting culture confirmation.7 The downstream consequences of antibiotics include potential adverse side effects, unnecessary healthcare costs, and further propagation of antimicrobial resistance trends.8
RADT workflow assessment
Over a period of 12 months from July 2022 through July 2023, all our clinics utilized an RADT when testing for Group A Streptococcus, a total of 22,382 tests. Culture confirmation of any negative result on patients less than 18 years of age is a requirement in our health system, aligning with the manufacturer and societal guidelines. Total negative RADTs on patients less than 18 years old amounted to 9,705 specimens.
Culture confirmation can quickly become complex as suspicious organisms require biochemical tests, further subculturing, or serogrouping. Consider the numerous workflow possibilities depicted in Figure 1 below. In total, our health system had 623 cultures return positive findings of Group A Streptococcus, a 10 percent false negative rate for the front-line RADT. Considering the highly active 2023 pharyngitis season, this was relatively poor performance.
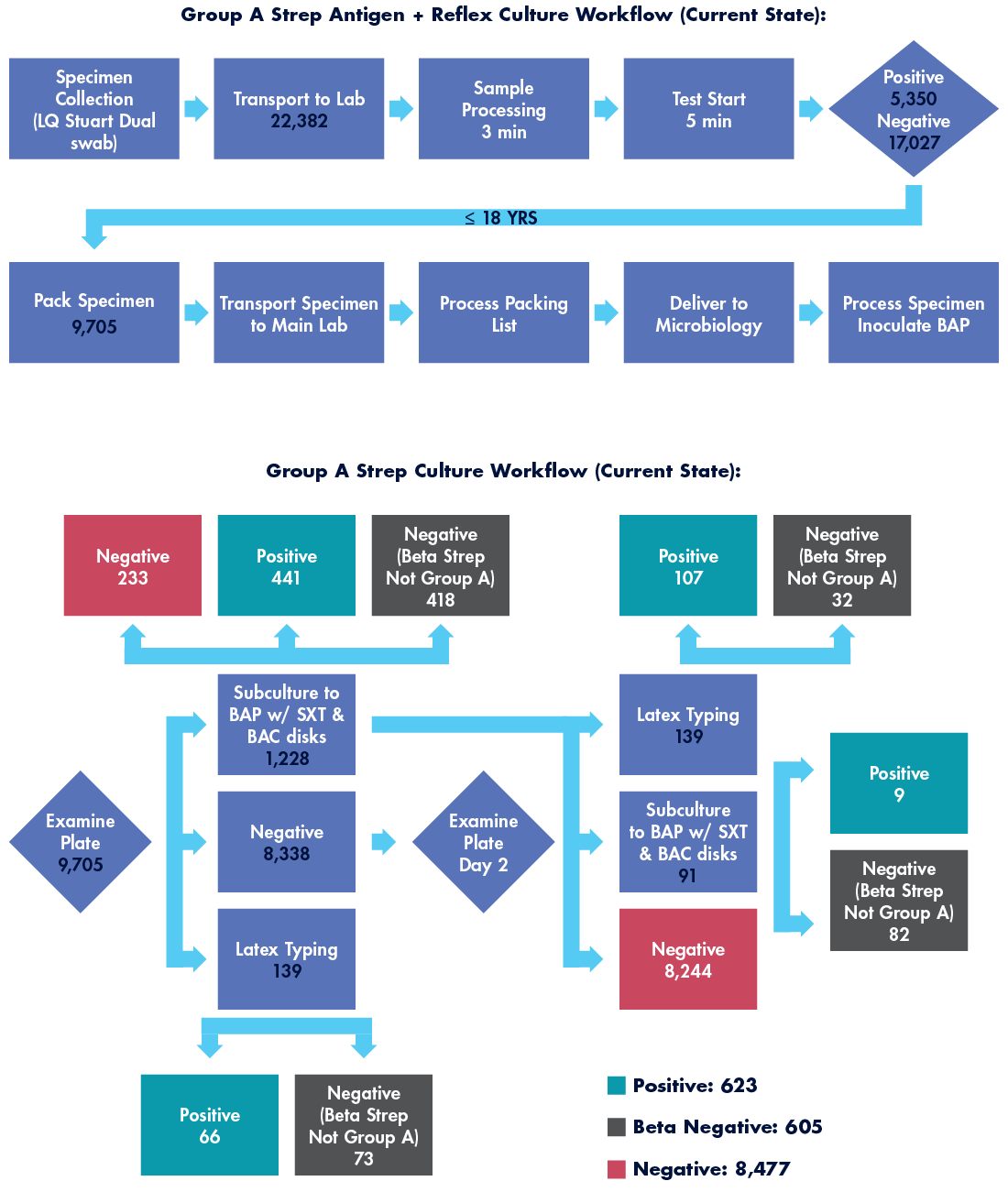
To determine the effort devoted to this laborious RADT with culture confirmation workflow, several staff members were timed under direct observation. Those times were averaged and multiplied against the total number of specimens cultured. In this workflow, total labor hours required to generate a finalized GAS test on 9,705 specimens generated over 346 labor hours annually.
Another important workflow consideration is the patient follow-up required when culture results are positive. Patients need to be informed of the finalized result and prescribed an antibiotic if not already initiated. For the callback process, we assigned each callback performed as five minutes of nurse labor time, generating an additional 52 hours of labor for the health system. On average, callbacks occur more than two days after specimen collection.
NAAT workflow assessment
Point-of-care NAATs are designed to contain minimal steps in test setup with moderately rapid (30 minutes or less) time to results. Many point-of-care (POC) NAATs only require three minutes or less of sample preparation, comparable to most RADTs. Assay turnaround time can range from less than 10 minutes to less than 45 minutes, possibly requiring a longer wait time for the provider and patient compared to an RADT. However, unlike RADT, the POC NAATs do not require confirmatory culture.
The workflow for POC NAATs for GAS is therefore minimal, requiring comparable labor for both specimen collection and test performance as an RADT. Additionally, as results are definitive upon completion, there are no patient callbacks or treatment changes required days after specimen collection.
Achievable benefits
Implementation of POC NAAT reduces numerous workflow steps, streamlining the process to definitive test results. By removing the various steps required to pack, transport, set up, interpret, and call back any relevant culture findings, a significant reduction in labor hours is achievable (see Figure 2 depicting labor hour comparison between workflows).
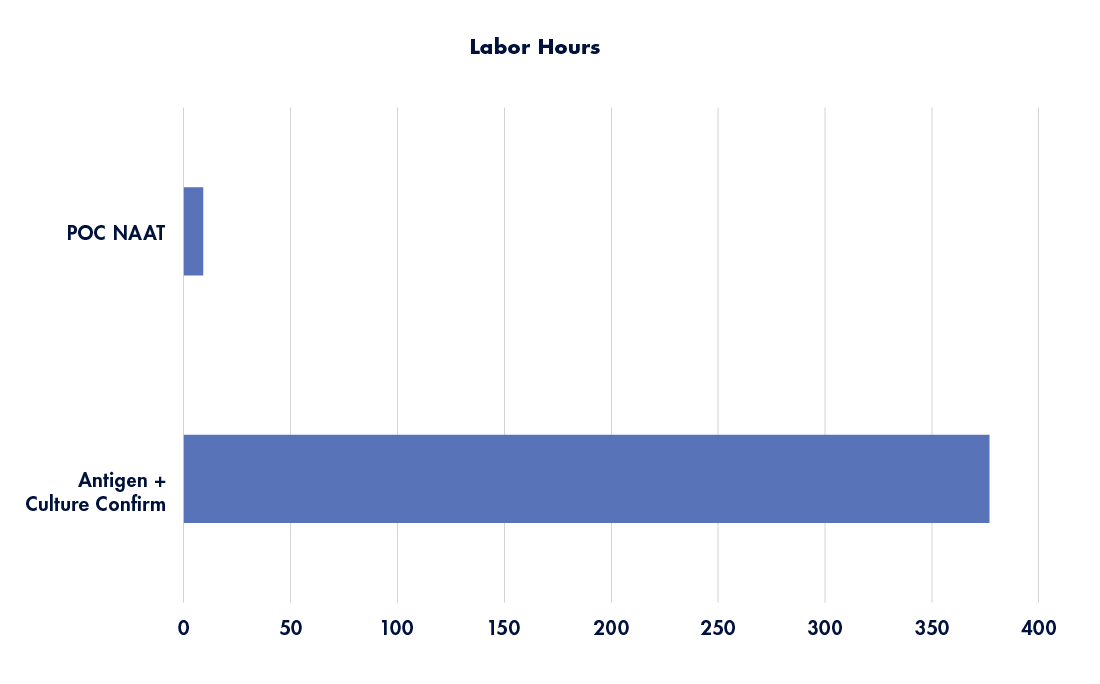
Figure 2: Annualized labor hours by workflow method. Source: Graphic recreated with data from Tyler Radke
Related webinar:
Get more insight on this topic during Tyler’s upcoming Lab Institute Virtual Event presentation, “Targeting Group A Streptococcus for System Improvement,” on June 5.
Visit www.g2intelligence.com/targeting-group-a-streptococcus-for-system-improvement/ for more details and to register.
Additionally, risks associated with specimen transport—such as lost specimens and deterioration of viable organism—are reduced.
By having real-time results, providers can appropriately treat (or not) the patient, without risk of unpleasant side effects or fear of downstream conditions.
Similarly, complex testing workloads in the microbiology lab are reduced, and the removal of patient callbacks gives numerous labor hours back to the lab and RN for more relevant care functions.
Finally, patient anxiety is reduced as there is no need to wait several days for culture confirmation. Parents with little ones, for example, are likely to be especially appreciative of the expediency and finality of the definitive NAAT result.
The time is now
Health systems that invested heavily in clinic-based NAAT platforms during the COVID-19 pandemic are uniquely positioned to reconsider current workflow practices. In the example of Group A Streptococcus, healthcare systems can capitalize on benefits to patients, providers, and the laboratory.
Many labs may be hesitant to expand molecular testing to the POC given the traditional lack of expertise in the ambulatory lab. However, in the case of Group A Streptococcus, POC molecular tests can yield labor efficiencies, result reliability, and improved turnaround time.
References:
Subscribe to view Essential
Start a Free Trial for immediate access to this article