
First Diagnostic Test for Long COVID Receives CE-IVD Marking
Based on validation study data, the test offers 90 percent accuracy and is unaffected by the emergence of new COVID-19 variants.

Based on validation study data, the test offers 90 percent accuracy and is unaffected by the emergence of new COVID-19 variants.
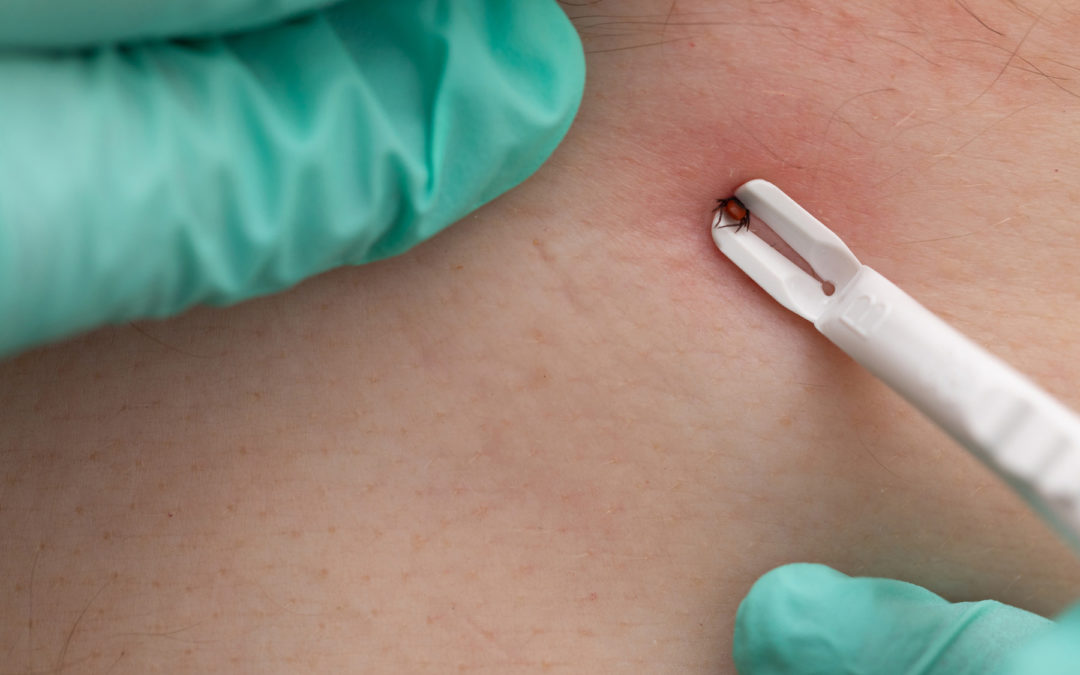
Recent study suggests a new test may help doctors detect Lyme disease during the early stages when treatment is likely to be more effective.

New Short-read Transpore Rapid Karyotyping test can identify fetal chromosomal abnormalities in a variety of samples.
Team creates a mass spectrometry-based blood lipid test that, when combined with AI, identifies early signs of the disease.
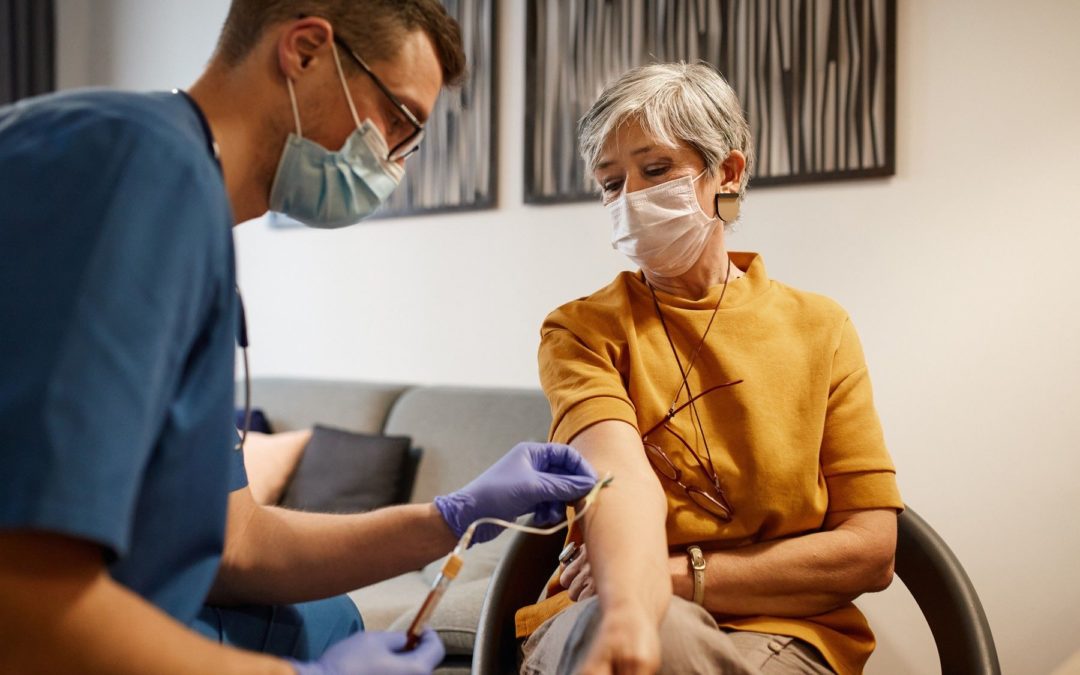
Recent developments suggest that we are on the precipice of a new age offering expanded options for Alzheimer’s diagnosis.