
The Benefits of Group A Streptococcus NAAT
Labs that invested heavily in POC molecular tests during COVID-19 are well positioned to leverage this equipment toward GAS diagnostics

Labs that invested heavily in POC molecular tests during COVID-19 are well positioned to leverage this equipment toward GAS diagnostics

Recent efforts to forecast the next infectious disease outbreak and steps labs can take to ensure they’re prepared.

Clinical Microbiology division head Jeff Fuller, FCCM, discusses his clinical laboratory’s recent automation transformation and offers tips to ensure success
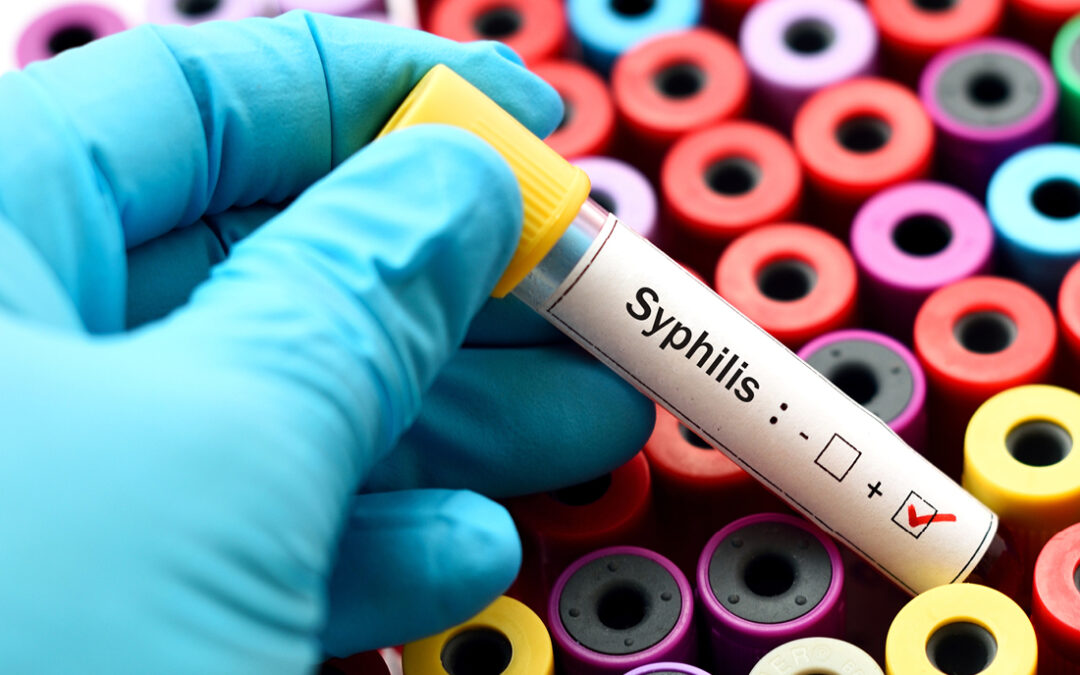
Key updates from the CDC’s new lab recommendations for syphilis testing.

Demand for at-home sample collection and infectious disease testing is at an all-time high—and still growing.