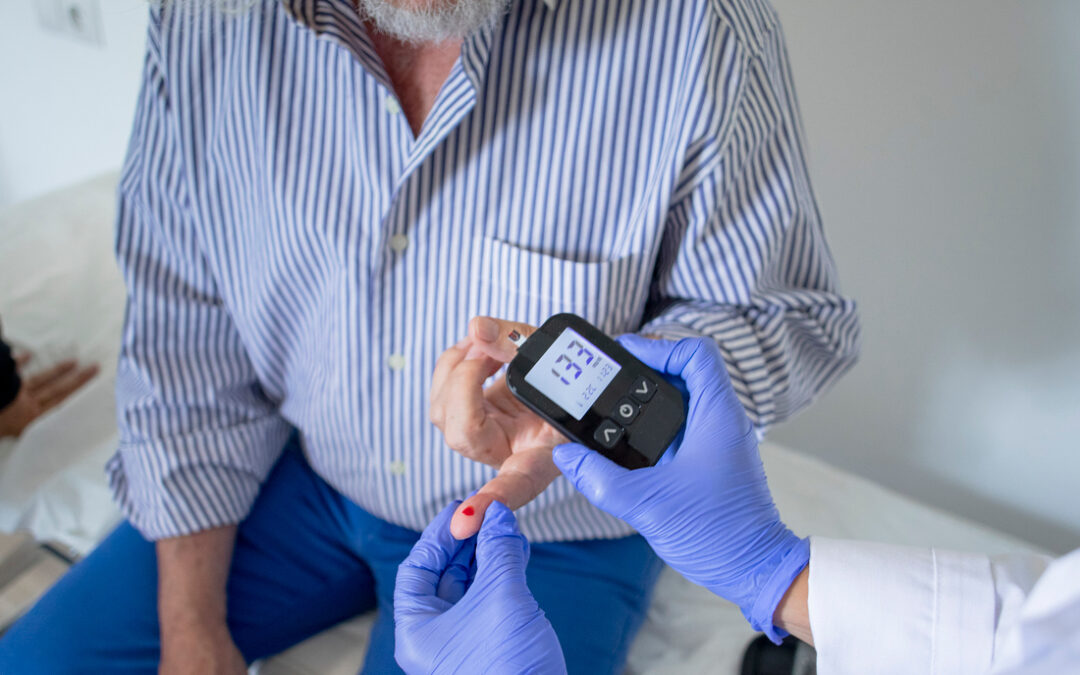
POC Testing: More FDA Approved Tests Than Ever, But Will Labs Be Reimbursed?
Revenue cycle management leaders provide their thoughts on how lab leaders may want to proceed when it comes to POC tests

Revenue cycle management leaders provide their thoughts on how lab leaders may want to proceed when it comes to POC tests

Patients desire direct-to-consumer testing, but are the results this testing provides accurate and reliable?

The grants, which include $3 million from the NIH and $1 million from the NSF, will help the company develop point-of-care tests
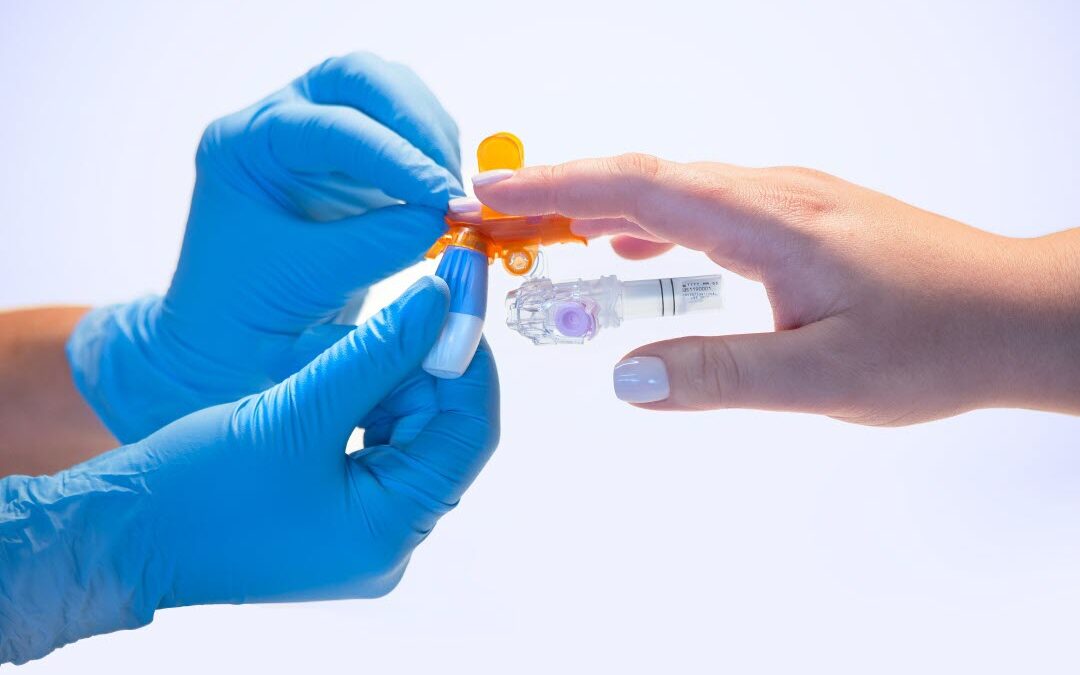
A new capillary blood collection system, the BD MiniDraw™, can improve the testing experience for both labs and patients

Labs that invested heavily in POC molecular tests during COVID-19 are well positioned to leverage this equipment toward GAS diagnostics